Nanofiltration (NF) and Reverse-Osmosis (RO) Membranes for Aqueous Ammonium Nitrate Salt Rejection: Experimental Studies †
Abstract
1. Introduction
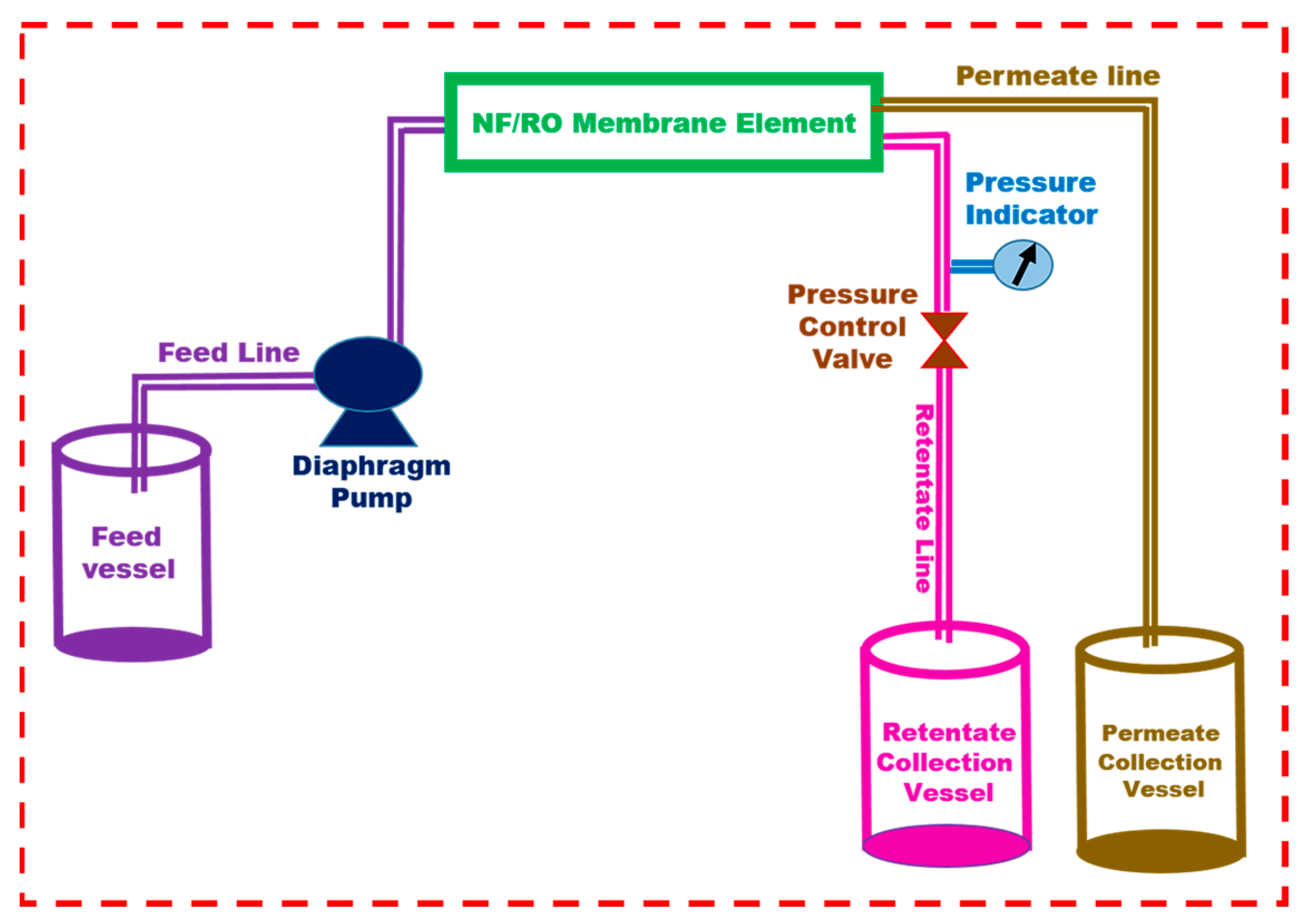
2. Materials and Methods
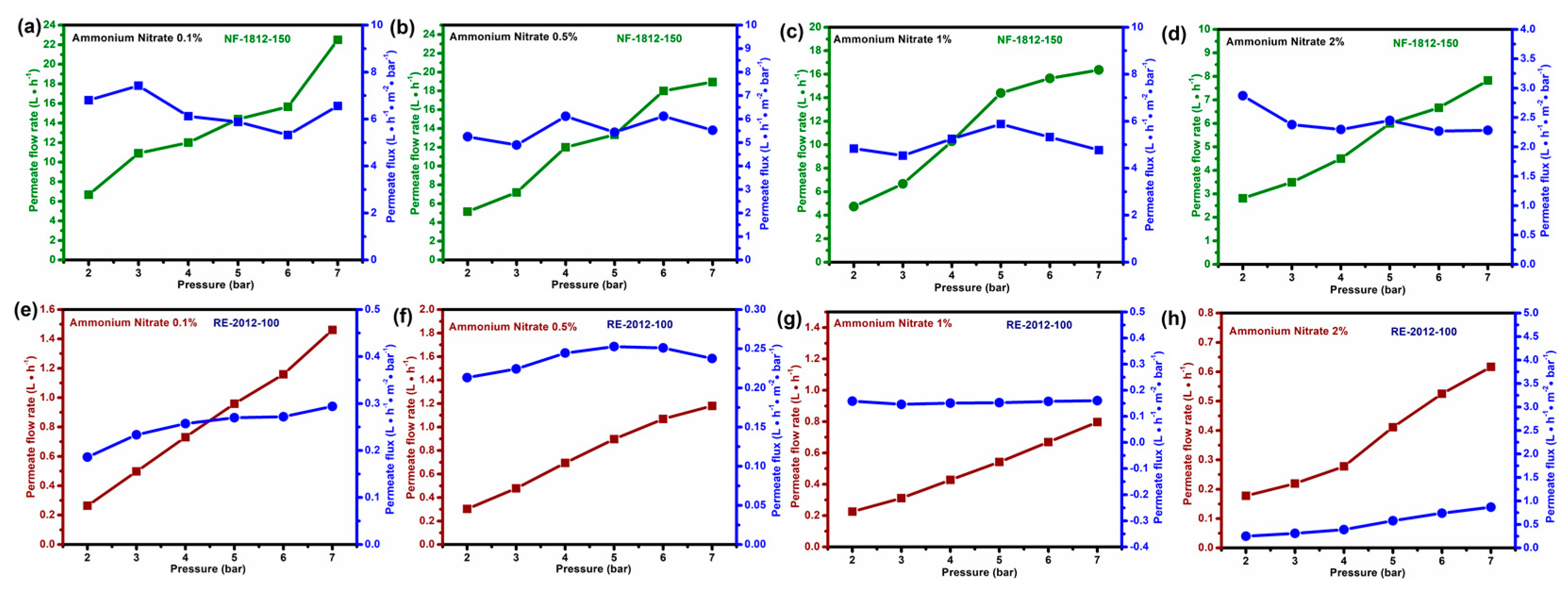
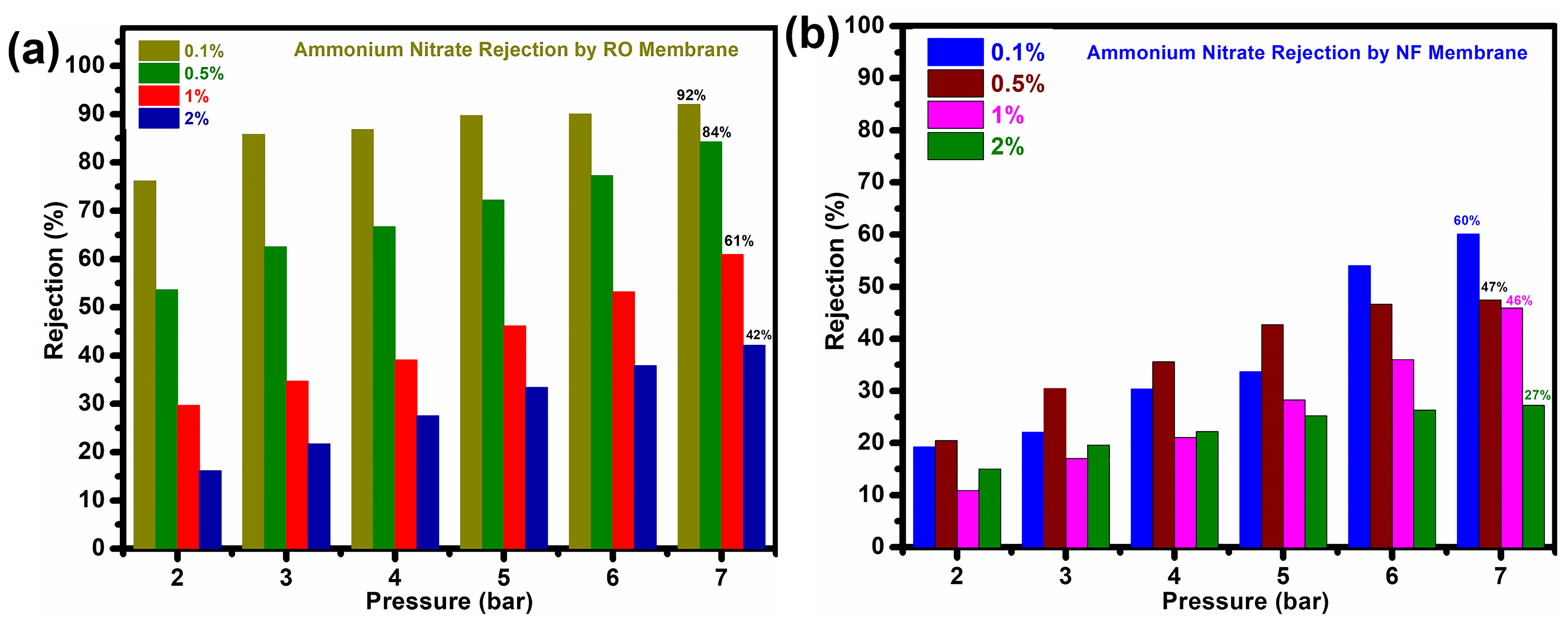
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, H.; Li, X.; Yang, W.; Yao, Z.; Mei, Y.; Peng, L.E.; Yang, Z.; Shao, S.; Tang, C.Y. Nanofiltration for drinking water treatment: A review. Front. Chem. Sci. Eng. 2022, 16, 681–698. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, Y.; Feng, Z.; Rui, X.; Zhang, T.; Zhang, Z. A Review on Reverse Osmosis and Nanofiltration Membranes for Water Purification. Polymers 2019, 11, 1252. [Google Scholar] [CrossRef] [PubMed]
- Warsinger, D.M.; Chakraborty, S.; Tow, E.W.; Plumlee, M.H.; Bellona, C.; Loutatidou, S.; Karimi, L.; Mikelonis, A.M.; Achilli, A.; Ghassemi, A.; et al. A review of polymeric membranes and processes for potable water reuse. Prog. Polym. Sci. 2016, 81, 209–237. [Google Scholar] [CrossRef]
- Wu, H.; Zhao, H.; Lin, Y.; Liu, X.; Wang, L.; Yao, H.; Tang, Y.; Yu, L.; Wang, H.; Wang, X. Positively-charged PEI/TMC nanofiltration membrane prepared by adding a diamino-silane coupling agent for Li+/Mg2+ separation. J. Membr. Sci. 2023, 672, 121468. [Google Scholar] [CrossRef]
- Tiruneh, A.A. Development in nanomembrane-based filtration of emerging contaminants. Phys. Sci. Rev. 2021, 8, 1659–1683. [Google Scholar] [CrossRef]
- Kumari, S.; Khan, A.A.; Chowdhury, A.; Bhakta, A.K.; Mekhalif, Z.; Hussain, S. Efficient and highly selective adsorption of cationic dyes and removal of ciprofloxacin antibiotic by surface modified nickel sulfide nanomaterials: Kinetics, isotherm and adsorption mechanism. Colloids. Surf. A Physicochem. Eng. Asp. 2020, 586, 124264. [Google Scholar] [CrossRef]
- Gholami, F.; Asadi, A.; Zinatizadeh, A.A. Efficient heavy metals and salts rejection using a novel modified polysul-fone nanofiltration membrane. Appl. Water Sci. 2022, 12, 146. [Google Scholar] [CrossRef]
- Zou, L.; Zhang, S.; Liu, J.; Cao, Y.; Qian, G.; Li, Y.Y.; Xu, Z.P. Nitrate removal from groundwater using negatively charged nanofiltration membrane. Environ. Sci. Pollut. Res. 2019, 26, 34197–34204. [Google Scholar] [CrossRef]
- Alzahrani, S.; Mohammad, A.W.; Hilal, N.; Abdullah, P.; Jaafar, O. Comparative study of NF and RO membranes in the treatment of produced water—Part I: Assessing water quality. Desalination 2013, 315, 18–26. [Google Scholar] [CrossRef]
| Specification | NF-1812-150 | RE-2012-100 |
|---|---|---|
| Material | Polyamide Thin Film | Polyamide Thin Film |
| Pore size | 2 nm–10 nm | 0.01 nm–0.1 nm |
| Diameter | 45.72 mm | 50.80 mm |
| Length | 304.8 mm | 304.8 mm |
| Effective surface area | 0.49 m2 | 0.71 m2 |
| Flow rate with (DMW) | 25 L·h−1 | 16.67 L·h−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, Z.; Qaisrani, T.M. Nanofiltration (NF) and Reverse-Osmosis (RO) Membranes for Aqueous Ammonium Nitrate Salt Rejection: Experimental Studies. Mater. Proc. 2024, 17, 20. https://doi.org/10.3390/materproc2024017020
Ali Z, Qaisrani TM. Nanofiltration (NF) and Reverse-Osmosis (RO) Membranes for Aqueous Ammonium Nitrate Salt Rejection: Experimental Studies. Materials Proceedings. 2024; 17(1):20. https://doi.org/10.3390/materproc2024017020
Chicago/Turabian StyleAli, Zulfiqar, and Tahir Maqsood Qaisrani. 2024. "Nanofiltration (NF) and Reverse-Osmosis (RO) Membranes for Aqueous Ammonium Nitrate Salt Rejection: Experimental Studies" Materials Proceedings 17, no. 1: 20. https://doi.org/10.3390/materproc2024017020
APA StyleAli, Z., & Qaisrani, T. M. (2024). Nanofiltration (NF) and Reverse-Osmosis (RO) Membranes for Aqueous Ammonium Nitrate Salt Rejection: Experimental Studies. Materials Proceedings, 17(1), 20. https://doi.org/10.3390/materproc2024017020






