Investigation of the Kinetics of the Adsorption of Methylene Blue on Activated Carbon †
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
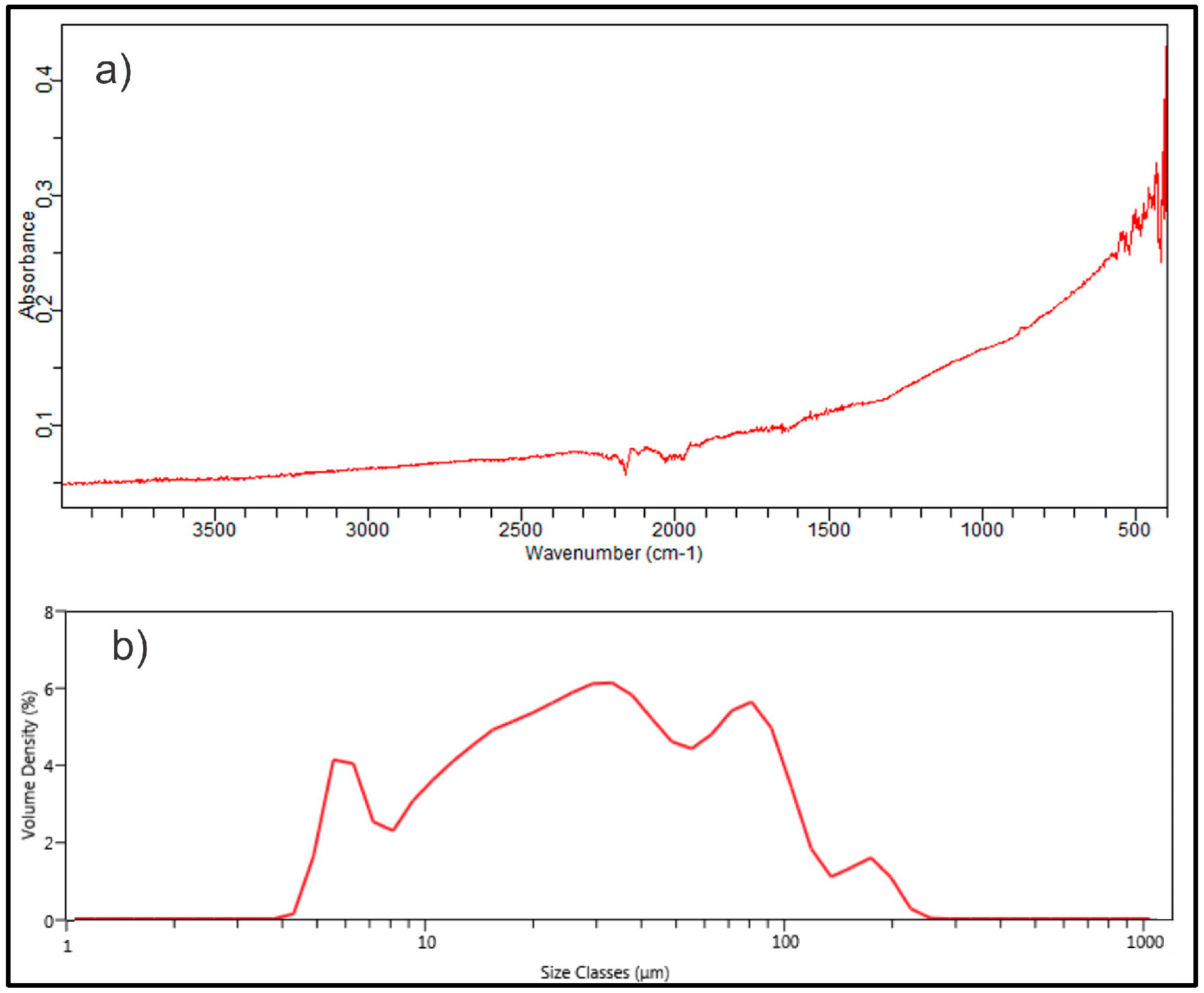
3.1. Adsorbent Characterization
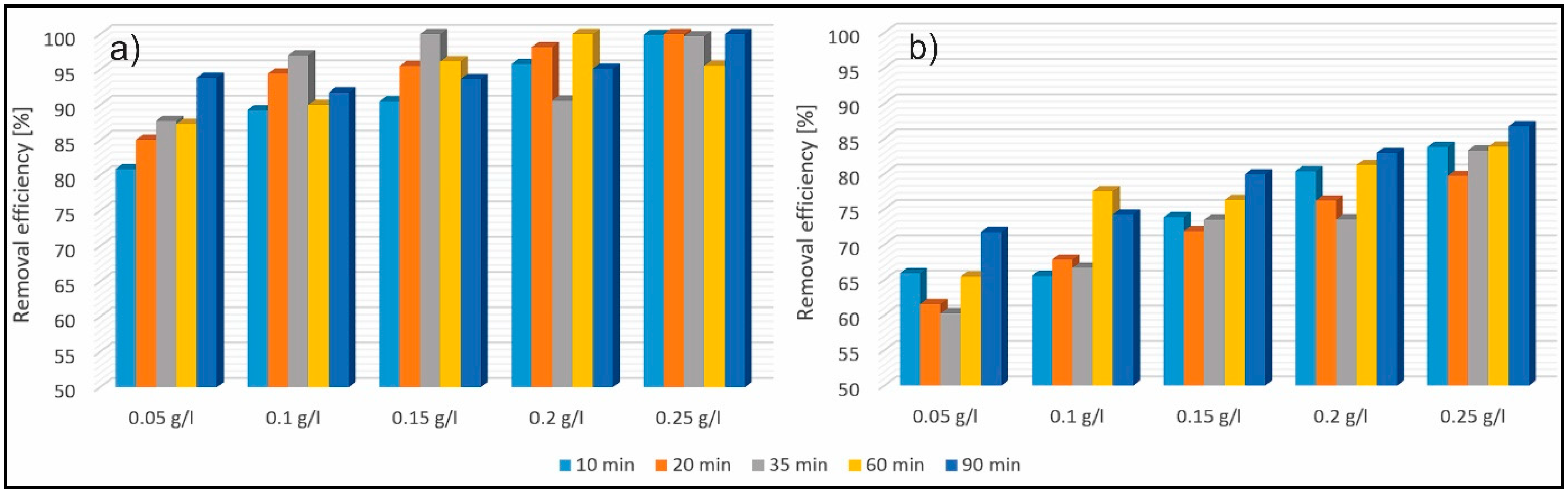
3.2. Investigation of the Removal Efficiency of Methylene Blue (MB)
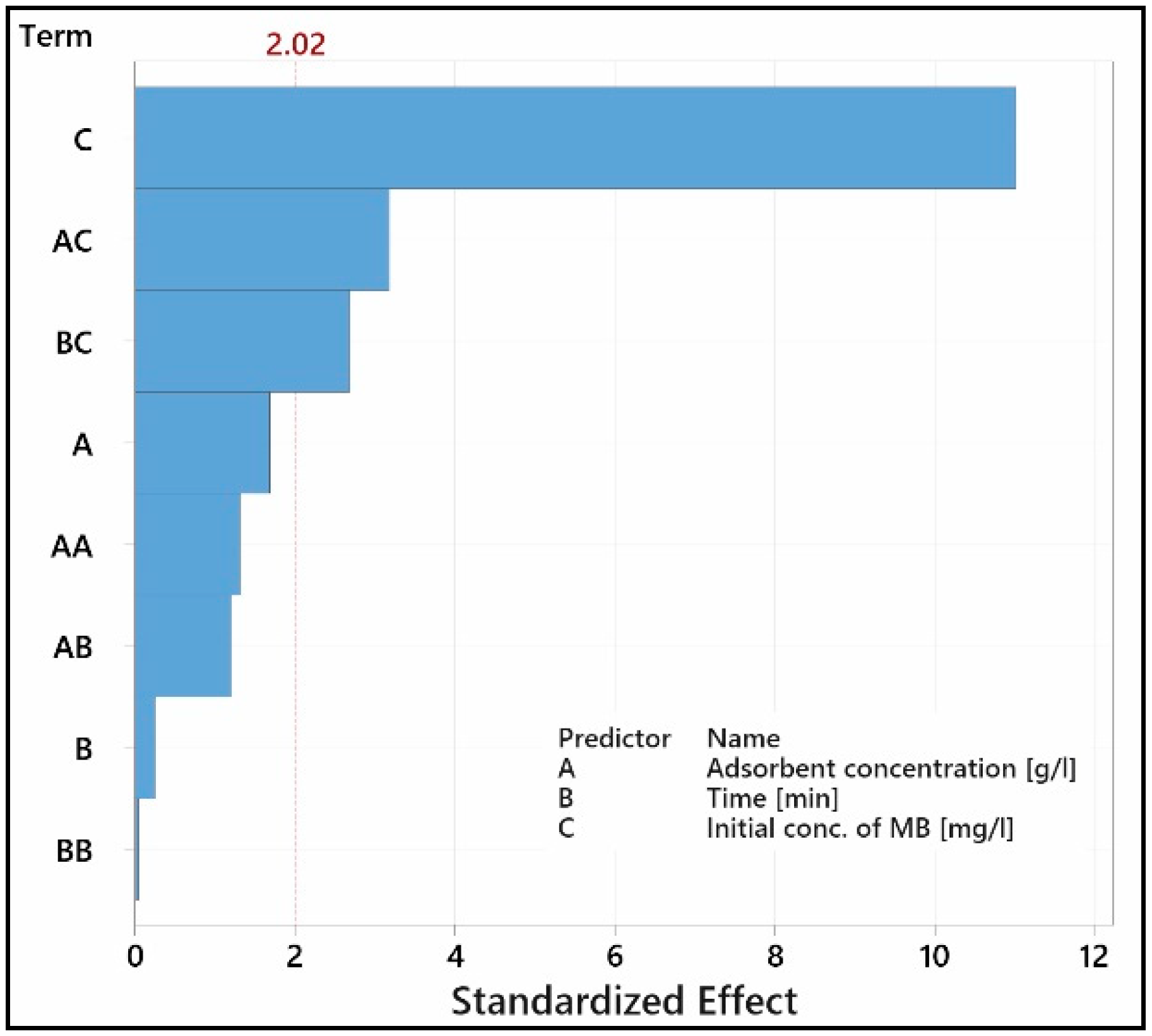
3.3. Investigation of Adsorption Kinetics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ramutshatsha-Makhwedzha, D.; Mavhungu, A.; Moropeng, M.L.; Mbaya, R. Activated Carbon Derived from Waste Orange and Lemon Peels for the Adsorption of Methyl Orange and Methylene Blue Dyes from Wastewater. Heliyon 2022, 8, e09930. [Google Scholar] [CrossRef] [PubMed]
- Kamdod, A.S.; Pavan Kumar, M.V. Adsorption of Methylene Blue and Methyl Orange on Tamarind Seed Activated Carbon and Its Composite with Chitosan: Equilibrium and Kinetic Studies. Desal. Water Treat. 2022, 252, 408–419. [Google Scholar] [CrossRef]
- Waisi, B.I.; Al-Furaiji, M.H.; McCutcheon, J.R. Activated Carbon Nanofibers Nonwoven Flat Sheet for Methylene Blue Dye Adsorption: Batch and Flow-through Systems. Desal. Water Treat. 2023, 289, 228–237. [Google Scholar] [CrossRef]
- Pan, X.; Zhao, N.; Shi, H.; Wang, H.; Ruan, F.; Wang, H.; Feng, Q. Biomass Activated Carbon Derived from Golden Needle Mushroom Root for the Methylene Blue and Methyl Orange Adsorption from Wastewater. Ind. Crops Prod. 2025, 223, 120051. [Google Scholar] [CrossRef]
- Mechati, F.; Djilani, C.; Bougdah, N.; Messikh, N.; Boussaha, E.; Moumen, A.; Bouchalta, C.; Medjram, M.S. Adsorption of Methylene Blue onto Activated Carbon Prepared under N2 /Microwave Radiation Supported Cobalt: Kinetics, Isotherms, and Thermodynamics Studies. Desal. Water Treat. 2023, 284, 288–300. [Google Scholar] [CrossRef]
- Somsiripan, T.; Sangwichien, C. Enhancement of Adsorption Capacity of Methylene Blue, Malachite Green, and Rhodamine B onto KOH Activated Carbon Derived from Oil Palm Empty Fruit Bunches. Arab. J. Chem. 2023, 16, 105270. [Google Scholar] [CrossRef]
- Jawad, A.H.; Sauodi, M.H.; Mastuli, M.S.; Aouda, M.A.; Radzun, K.A. Pomegranate Peels Collected from Fresh Juice Shop as a Renewable Precursor for High Surface Area Activated Carbon with Potential Application for Methylene Blue Adsorption. Desal. Water Treat. 2018, 124, 287–296. [Google Scholar] [CrossRef]
- Dimbo, D.; Abewaa, M.; Adino, E.; Mengistu, A.; Takele, T.; Oro, A.; Rangaraju, M. Methylene Blue Adsorption from Aqueous Solution Using Activated Carbon of Spathodea campanulata. Results Eng. 2024, 21, 101910. [Google Scholar] [CrossRef]
- Khebli, Z.; Djardire, R.; Boulkrinat, A.; Bouzerara, F. Elaboration of Zirconia Microfiltration Membranes Tested with Methylene Blue Filtration. Desal. Water Treat. 2022, 279, 4–7. [Google Scholar] [CrossRef]
- Sotelo, S.; Oyarce, E.; Roa, K.; Boulett, A.; Pizarro, G.; Sánchez, J. Sodium Lignosulfonate as an Extracting Agent of Methylene Blue Dye Using a Polymer-Enhanced Ultrafiltration Technique. Int. J. Biol. Macromol. 2024, 275, 133567. [Google Scholar] [CrossRef]
- Moghazy, R.M.; Mahmoud, R.H. Microalgal-Based Macro-Hollow Loofah Fiber Bio-Composite for Methylene Blue Removal: A Promising Step for a Green Adsorbent. Int. J. Biol. Macromol. 2023, 253, 127009. [Google Scholar] [CrossRef] [PubMed]
- Ihaddaden, S.; Aberkane, D.; Boukerroui, A.; Robert, D. Removal of Methylene Blue (Basic Dye) by Coagulation-Flocculation with Biomaterials (Bentonite and Opuntia ficus indica). J. Water Process Eng. 2022, 49, 102952. [Google Scholar] [CrossRef]
- Jiao, R.; Lou, T.; Zhang, H.; Wang, X. Preparation of Starch-Acrylic Acid-Carboxymethyl Cellulose Copolymer and Its Flocculation Performance towards Methylene Blue. Biochem. Eng. J. 2022, 187, 108635. [Google Scholar] [CrossRef]
- Hu, Z.; Guo, C.; Wang, P.; Guo, R.; Liu, X.; Tian, Y. Electrochemical Degradation of Methylene Blue by Pb Modified Porous SnO2 Anode. Chemosphere 2022, 305, 135447. [Google Scholar] [CrossRef]
- Settu, M.; Gnanamoorthy, G.; Thirugnanam, B.; Vengidusamy, N.; Alotaibi, M.A. Vanadium Nitride /Poly(0-Methoxy Aniline)- Poly(3,4-Ethylene Dioxythiophene) Interpenetrated into Nanocomposite for Efficient Photocatalytic Degradation of Methylene Blue and Enhanced Electrochemical Sensing of Mebendazole. Biosens. Bioelectron. X 2024, 19, 100508. [Google Scholar] [CrossRef]
- Hadi, S.; Taheri, E.; Amin, M.M.; Fatehizadeh, A.; Gardas, R.L. Empirical Modeling and Kinetic Study of Methylene Blue Removal from Synthetic Wastewater by Activation of Persulfate with Heterogeneous Fenton-like Process. J. Mol. Liq. 2021, 328, 115408. [Google Scholar] [CrossRef]
- Ma, D.Y.; Wang, X.H.; Song, C.; Wang, S.G.; Fan, M.H.; Li, X.M. Aerobic Granulation for Methylene Blue Biodegradation in a Sequencing Batch Reactor. Desalination 2011, 276, 233–238. [Google Scholar] [CrossRef]
- Liu, W.; Liu, L.; Liu, C.; Hao, Y.; Yang, H.; Yuan, B.; Jiang, J. Methylene Blue Enhances the Anaerobic Decolorization and Detoxication of Azo Dye by Shewanella Onediensis MR-1. Biochem. Eng. J. 2016, 110, 115–124. [Google Scholar] [CrossRef]
- Khan, M.; Ahmed, M.M.; Akhtar, M.N.; Sajid, M.; Riaz, N.N.; Asif, M.; Kashif, M.; Shabbir, B.; Ahmad, K.; Saeed, M.; et al. Fabrication of CuWO4@MIL-101 (Fe) Nanocomposite for Efficient OER and Photodegradation of Methylene Blue. Heliyon 2024, 10, e40546. [Google Scholar] [CrossRef]
- Balavinoth, M.; Sumithraj Premkumar, P.; Ravi Samuel Raj, C.; Ilavarasi Jeyamalar, J. Synthesis and Characterization of Mesoporous MCM-41 from Oil Template for Effective Adsorption of Methylene Blue Dye. Phosphorus Sulfur Silicon Relat. Elem. 2025, 200, 68–76. [Google Scholar] [CrossRef]
- Dwivedi, P.; Rathore, A.K.; Srivastava, D.; Vijayakumar, R.P. Mechanistic Impact of Sodium Nitrate on the Characteristics of MWCNTS Oxidation and Potential Application on Methylene Blue Adsorption from Wastewater. Waste Manag. Bull. 2025, 3, 207–218. [Google Scholar] [CrossRef]
- Misran, E.; Supardan, M.D.; Iryani, D.A.; Pramananda, V.; Sihombing, A.F.; Sitorus, D.V. Ultrasonic Assisted Adsorption of Methylene Blue Using Blood Clam Shell as a Low-Cost Adsorbent. Results Eng. 2024, 23, 102715. [Google Scholar] [CrossRef]
- Borghei, S.A.; Zare, M.H.; Ahmadi, M.; Sadeghi, M.H.; Marjani, A.; Shirazian, S.; Ghadiri, M. Synthesis of Multi-Application Activated Carbon from Oak Seeds by KOH Activation for Methylene Blue Adsorption and Electrochemical Supercapacitor Electrode. Arab. J. Chem. 2021, 14, 102958. [Google Scholar] [CrossRef]
- Francoeur, M.; Yacou, C.; Jean-Marius, C.; Chérémond, Y.; Jauregui-Haza, U.; Gaspard, S. Optimization of the Synthesis of Activated Carbon Prepared from Sargassum (sp.) and Its Use for Tetracycline, Penicillin, Caffeine and Methylene Blue Adsorption from Contaminated Water. Environ. Technol. Innov. 2022, 28, 102940. [Google Scholar] [CrossRef]
- Iqajtaoune, A.; Taibi, M.; Saufi, H.; Aouan, B.; Boudad, L. Enhanced Removal of Methylene Blue and Procion Deep Red H-EXL Dyes from Aqueous Environments by Modified-Bentonite: Isotherm, Kinetic, and Thermodynamic. Desal. Water Treat. 2024, 320, 100607. [Google Scholar] [CrossRef]
- Hayfron, J.; Jääskeläinen, S.; Tetteh, S. Synthesis of Zeolite from Rice Husk Ash and Kaolinite Clay for the Removal of Methylene Blue from Aqueous Solution. Heliyon 2025, 11, e41325. [Google Scholar] [CrossRef]
- Al-Marri, A.H.; Moulahi, A.; Mogharbel, A.T.; Al-Mohaimeed, A.M.; Janene, F.; Ouda, A.S.; Al-Farraj, E.S.; Almaslamani, M.N.; Almaslamani, M.A.; Mjejri, I. Photocatalytic Performance of Nb2O5-Graphene Heterojunction for the Degradation of Methylene Blue. Polyhedron 2024, 260, 117080. [Google Scholar] [CrossRef]
- Dolas, H. Activated Carbon Synthesis and Methylene Blue Adsorption from Pepper Stem Using Microwave Assisted Impregnation Method: Isotherm and Kinetics. J. King Saud Univ. Sci. 2023, 35, 102559. [Google Scholar] [CrossRef]
- Zakaria, R.; Jamalluddin, N.A.; Abu Bakar, M.Z. Effect of Impregnation Ratio and Activation Temperature on the Yield and Adsorption Performance of Mangrove Based Activated Carbon for Methylene Blue Removal. Results Mater. 2021, 10, 102559. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, J.; Li, Y.; Zhang, P.; Li, M.; Zheng, H.; Zhang, X.; Li, H.; Du, Q. Methylene Blue Adsorption by Activated Carbon, Nickel Alginate/Activated Carbon Aerogel, and Nickel Alginate/Graphene Oxide Aerogel: A Comparison Study. J. Mater. Res. Technol. 2020, 9, 12443–12460. [Google Scholar] [CrossRef]
- Bih, N.L.; Rwiza, M.J.; Ripanda, A.S.; Mahamat, A.A.; Machunda, R.L.; Choi, J.W. Adsorption of Phenol and Methylene Blue Contaminants onto High-Performance Catalytic Activated Carbon from Biomass Residues. Heliyon 2025, 11, e41150. [Google Scholar] [CrossRef] [PubMed]
- Medhat, A.; El-Maghrabi, H.H.; Abdelghany, A.; Abdel Menem, N.M.; Raynaud, P.; Moustafa, Y.M.; Elsayed, M.A.; Nada, A.A. Efficiently Activated Carbons from Corn Cob for Methylene Blue Adsorption. Appl. Surf. Sci. Adv. 2021, 3, 100037. [Google Scholar] [CrossRef]
- Oun, A.A.; Kamal, K.H.; Farroh, K.; Ali, E.F.; Hassan, M.A. Development of Fast and High-efficiency Sponge-gourd Fibers (Luffa Cylindrica)/Hydroxyapatite Composites for Removal of Lead and Methylene Blue. Arab. J. Chem. 2021, 14, 103281. [Google Scholar] [CrossRef]
- Lagergren, S. Zur Theorie der sogenannten Adsorption gelöster Stoffe. Z. Chem. Ind. Kolloide 1907, 2, 15. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second Order Model for Sorption Processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Hussain, O.A.; Hathout, A.S.; Abdel-Mobdy, Y.E.; Rashed, M.M.; Abdel Rahim, E.A.; Fouzy, A.S.M. Preparation and Characterization of Activated Carbon from Agricultural Wastes and their Ability to Remove Chlorpyrifos from Water. Toxicol. Rep. 2023, 10, 146–154. [Google Scholar] [CrossRef]
- Verdoorn, D.S.; Zuliani, A.; Ranjan, P.J.; Holgado, P.; Khiar, N.; Saya, J.M.; Carrillo-Carrión, C.; Maes, B.U.W.; Orru, R.V.A. Unveiling the Potential of a Cobalt-Based Metal-Organic Framework in Carbodiimide Synthesis. Adv. Synth. Catal. 2025, 367, e202401540. [Google Scholar] [CrossRef]
- Miura, H.; Sasaki, S.; Ogawa, R.; Shishido, T. Hydrosilylation of Allenes Over Palladium–Gold Alloy Catalysts: Enhancing Activity and Switching Selectivity by the Incorporation of Palladium into Gold Nanoparticles. Eur. J. Org. Chem. 2018, 2018, 1858–1862. [Google Scholar] [CrossRef]
- Kaczmarski, K.; Przywara, M.; Lorenc-Grabowska, E. Advanced Modelling of Adsorption Process on Activated Carbon. Chem. Eng. Res. Des. 2022, 181, 27–40. [Google Scholar] [CrossRef]
- Mustapha, L.S.; Kolade, S.O.; Durosinmi, S.O.; Tan, I.S.; Lau, S.Y.; Obayomi, K.S. Anthill Clay Activated Ocimum Gratissimum Extract for Effective Adsorption of Methylene Blue and Chromium (VI) Ion from Wastewater: Insights into the Adsorption Isotherms, Kinetics, Thermodynamics, and Mechanisms. J. Water Process. Eng. 2024, 67, 106286. [Google Scholar] [CrossRef]
- Rao Vaddi, D.; Malla, R.; Geddapu, S. Magnetic Activated Carbon: A Promising Approach for the Removal of Methylene Blue from Wastewater. Desal. Water Treat. 2024, 317, 100146. [Google Scholar] [CrossRef]
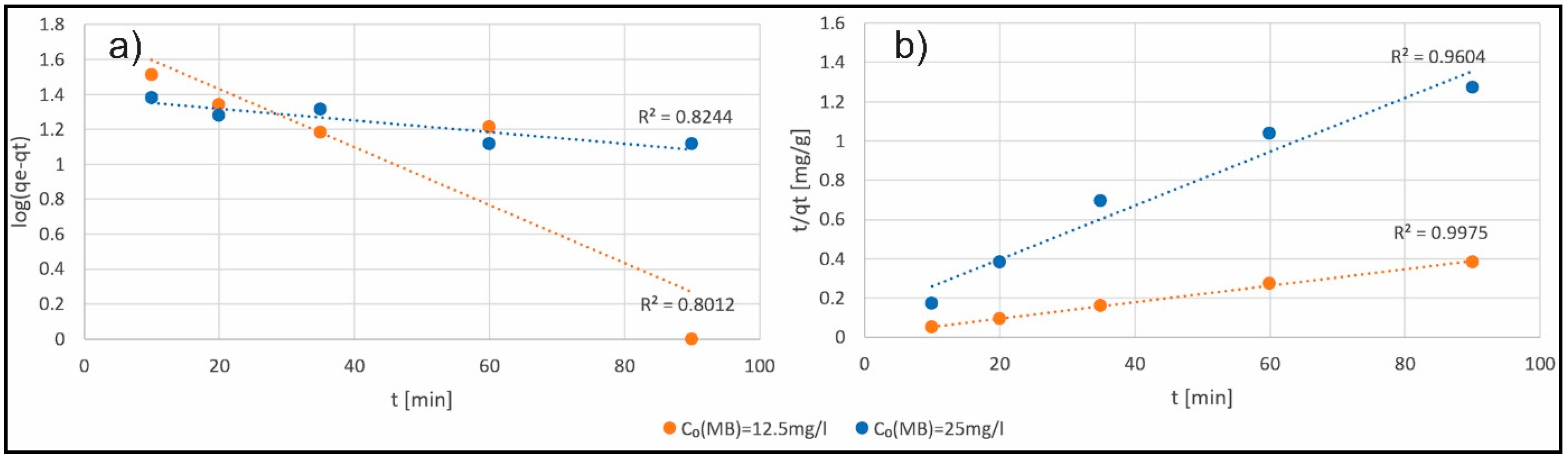

| Adsorbent Conc. [g/L] | Equation | Slope | Intercept | qe [mg/g] | k2 [g/(mg·min)] | R2 |
|---|---|---|---|---|---|---|
| 12.5 mg/L | ||||||
| 0.05 | t/qt = 0.0110 + 0.0042t | 0.0042 | 0.0110 | 238.10 | 0.00160 | 0.9975 |
| 0.1 | t/qt = 0.0052 + 0.0088t | 0.0088 | 0.0052 | 113.64 | 0.01489 | 0.9989 |
| 0.15 | t/qt = 0.0084 + 0.0128t | 0.0128 | 0.0084 | 78.13 | 0.01950 | 0.9989 |
| 0.2 | t/qt = 0.0018 + 0.0166t | 0.0166 | 0.0018 | 60.24 | 0.15309 | 0.9974 |
| 0.25 | t/qt = 0.0022 + 0.0202t | 0.0202 | 0.0022 | 49.50 | 0.18547 | 0.9987 |
| 25 mg/L | ||||||
| 0.05 | t/qt = 0.1220 + 0.0137t | 0.0137 | 0.1220 | 72.99 | 0.00154 | 0.9604 |
| 0.1 | t/qt = 0.1720 + 0.0231t | 0.0231 | 0.1720 | 43.29 | 0.00310 | 0.9622 |
| 0.15 | t/qt = 0.2571 + 0.0288t | 0.0288 | 0.2571 | 34.72 | 0.00323 | 0.9774 |
| 0.2 | t/qt = 0.3389 + 0.0317t | 0.0317 | 0.3389 | 31.55 | 0.00297 | 0.9437 |
| 0.25 | t/qt = 0.1708 + 0.0365t | 0.0365 | 0.1708 | 27.40 | 0.00780 | 0.9934 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasiljević, N.; Panić, S.; Tadić, G.; Vuković, J.; Novaković, N.; Mićić, V. Investigation of the Kinetics of the Adsorption of Methylene Blue on Activated Carbon. Eng. Proc. 2025, 99, 4. https://doi.org/10.3390/engproc2025099004
Vasiljević N, Panić S, Tadić G, Vuković J, Novaković N, Mićić V. Investigation of the Kinetics of the Adsorption of Methylene Blue on Activated Carbon. Engineering Proceedings. 2025; 99(1):4. https://doi.org/10.3390/engproc2025099004
Chicago/Turabian StyleVasiljević, Nebojša, Sanja Panić, Goran Tadić, Jelena Vuković, Nataša Novaković, and Vladan Mićić. 2025. "Investigation of the Kinetics of the Adsorption of Methylene Blue on Activated Carbon" Engineering Proceedings 99, no. 1: 4. https://doi.org/10.3390/engproc2025099004
APA StyleVasiljević, N., Panić, S., Tadić, G., Vuković, J., Novaković, N., & Mićić, V. (2025). Investigation of the Kinetics of the Adsorption of Methylene Blue on Activated Carbon. Engineering Proceedings, 99(1), 4. https://doi.org/10.3390/engproc2025099004







