Assessment of the Oxidative State of Thermally Treated Sunflower Oil After Regeneration with Molecular Sieves †
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dimić, E.; Turkulov, J. Kontrola kvaliteta u tehnologiji jestivih ulja; Tehnološki fakultet: Novi Sad, Serbia, 2000. [Google Scholar]
- Fadda, A.; Sanna, D.; Sakar, E.H.; Gharby, S.; Mulas, M.; Medda, S.; Yesilcubuk, N.S.; Karaca, A.C.; Gozukirmizi, C.K.; Lucarini, M.; et al. Innovative and sustainable technologies to enhance the oxidative stability of vegetable oils. Sustainability 2022, 14, 849. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Chemistry of Deep-Fat Frying Oils. J. Food Sci. 2007, 72, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Wonglamom, J.; Rakariyatham, N. Recovery of used vegetable frying oil by two step adsorbents. In Proceedings of the 26th Annual Meeting of the Thai Society for Biotechnology and International Conference, Chiang Mai, Thailand, 26–29 November 2014; pp. 330–337. [Google Scholar]
- Dobrnjac, S.; Dobrnjac, M.; Skundric, J.P.; Vasiljević, L.; Blagojević, S.; Sandic, Z. Possibility for Removing Products of Thermal Degradation of Edible Oil by Natural Aluminosilicates. In Proceedings of the International Conference of Experimental and Numerical Investigations and New Technologies, CNNTech 2018; Springer: Berlin/Heidelberg, Germany, 2018; Volume 54, pp. 59–71. [Google Scholar]
- Dobrnjac, S.; Vasiljević, L.; Blagojević, S.; Gligorić, M.; Obrenović, Z.; Cvijetinović, V.; Tošković, D. Removing Products of Thermal Degradation from Edible Oils by Zeolite and by Clinoptilolite—Comparison of Results. In Proceedings of the VI International Congress Engineering, Environment and Materials in Processing Industry, Jahorina, Bosnia and Herzegovina, 11–13 March 2019; pp. 384–392. [Google Scholar]
- Hudson, B.J.F.; Gordon, M.H. Rancidity in Foods. In Rancidity in Foods, 3rd ed.; Allen, J.C., Hamilton, R.J., Eds.; Applied Science: Barking, UK, 1994; Chapter 3. [Google Scholar]
- Robards, K.; Kerr, A.F.; Patsalides, E. Rancidity and its measurement in edible oils and snack foods: A review. Analyst 1988, 113, 213. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Cuevas, L.; Castellano, G.; Torrens, F.; Raikos, V. Revealing the relationship between vegetable oil composition and oxidative stability: A multifactorial approach. J. Food Compos. Anal. 2018, 66, 221–229. [Google Scholar] [CrossRef]
- Penavin, J. Izomerizacija 3,3 dimetilbutena 1 na zeolitima. Master’s Thesis, University of Zagreb, Zagreb, Croatia, 1979. [Google Scholar]
- Penavin, J.; Škundrić, J.; Levi, Z.; Sladojević, S.; Škundrić, B.; Čegar, N.; Šušnjar, L.; Sredić, S. Mogućnost primjene tufova sa lokaliteta Republike Srpske kao adsorbenasa za kiselo-bazne primjese u otpadnim vodama. In Proceedings of the EcoIst’05, Ekološka Istina, Bor, Serbia, 1–4 June 2005. [Google Scholar]
- Sladojević, S.; Penavin-Škundrić, J.; Škundrić, B.; Lazić, D.; Krnetić, S.; Vujasinović, S.; Zeljković, S. Uticaj sastava i strukture zeolita na njegove adsorpcione osobine. In Proceedings of the VII Naučno/stručni simpozijum sa međunarodnim učešćem Metalni i nemetalni materijali, Zenica, Bosnia and Herzegovina, 22–23 May 2008; pp. 423–428. [Google Scholar]
- Rajić, D.; Vasiljević, L.; Tošković, D.; Gligorić, M.; Obrenović, Z. Sinteza i karakterizacija alumosilikata kao potencijalnog adsorbensa dibutilftalata. In Proceedings of the V International Congress Engineering, Environment and Materials in Processing Industry, Jahorina, Bosnia and Herzegovina, 15–17 March 2017; pp. 1521–1530, ISBN 978-99955-81-21-3. [Google Scholar]
- Vasiljević, L. Modeliranje sinteze i karakterizacija zeolita tipa A u cilju poboljšanja njegovih osobina. Ph.D. Thesis, Prirodno-matematički fakultet Banja Luka, Banja Luka, Bosnia and Herzegovina, 2009. [Google Scholar]
- Broach, R.W.; Jan, D.-Y.; Lesch, D.A.; Kulprathipanja, S.; Roland, E.; Kleinschmit, P. Zeolites. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Hoboken, NJ, USA, 2012. [Google Scholar]
- Ayuso, E.A.; Sanchez, A.G.; Querol, X. Purification of metal electroplating waste waters using zeolites. Water Res. 2003, 37, 4855–4862. [Google Scholar] [CrossRef] [PubMed]
- Colella, C.; Wise, W.S. The IZA Handbook of Natural Zeolites: A tool of knowledge on the most important family of porous minerals. Microporous Mesoporous Mater. 2014, 189, 4–10. [Google Scholar] [CrossRef]
- Godelitsas, A.; Armbruster, T. HEU-type zeolites modified by transition elements and lead. Microporous Mesoporous Mater. 2003, 61, 3–24. [Google Scholar] [CrossRef]
- Shia, W.Y.; Shaoa, H.B.; Li, H.; Shaoa, M.A.; Dua, S. Progress in the remediation of hazardous heavy metal-polluted soils by natural zeolite. J. Hazard. Mater. 2009, 170, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, A.S.; Sakaria, P.L.; Vasudevan, M.; Pawar, R.R.; Sudheesh, N.; Bajaj, H.C.; Mody, H.M. Adsorption of an anionic dye from aqueous medium by organoclays: Equilibrium modeling, kinetic and thermodynamic exploration. RSC Adv. 2012, 2, 8663–8671. [Google Scholar] [CrossRef]
- Patel, H.A.; Joshi, G.V.; Pawar, R.R.; Bajaj, H.C.; Jasra, R.V. Mechanical and thermal properties of polypropylene nanocomposites using organically modified Indian bentonite. Polym. Compos. 2010, 31, 399–404. [Google Scholar] [CrossRef]
- Wang, S.; Dong, Y.; He, M.; Chen, L.; Yu, X. Characterization of GMZ bentonite and its application in the adsorption of Pb(II) from aqueous solutions. Appl. Clay Sci. 2009, 43, 164–171. [Google Scholar] [CrossRef]
- Suuronen, J.-P.; Matusewicz, M.; Olin, M.; Serimaa, R. X-ray studies on the nano- and microscale anisotropy in compacted clays: Comparison of bentonite and purified calcium montmorillonite. Appl. Clay Sci. 2014, 101, 401–408. [Google Scholar] [CrossRef]
- Kul, A.R.; Koyuncu, H.J. Adsorption of Pb(II) ions from aqueous solution by native and activated bentonite: Kinetic, equilibrium and thermodynamic study. Hazard. Mater. 2010, 179, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Bergaya, F.; Lagaly, G. Developments in Clay Science; Elsevier: Amsterdam, The Netherlands, 2006; Volume 1, pp. 1–18. [Google Scholar]
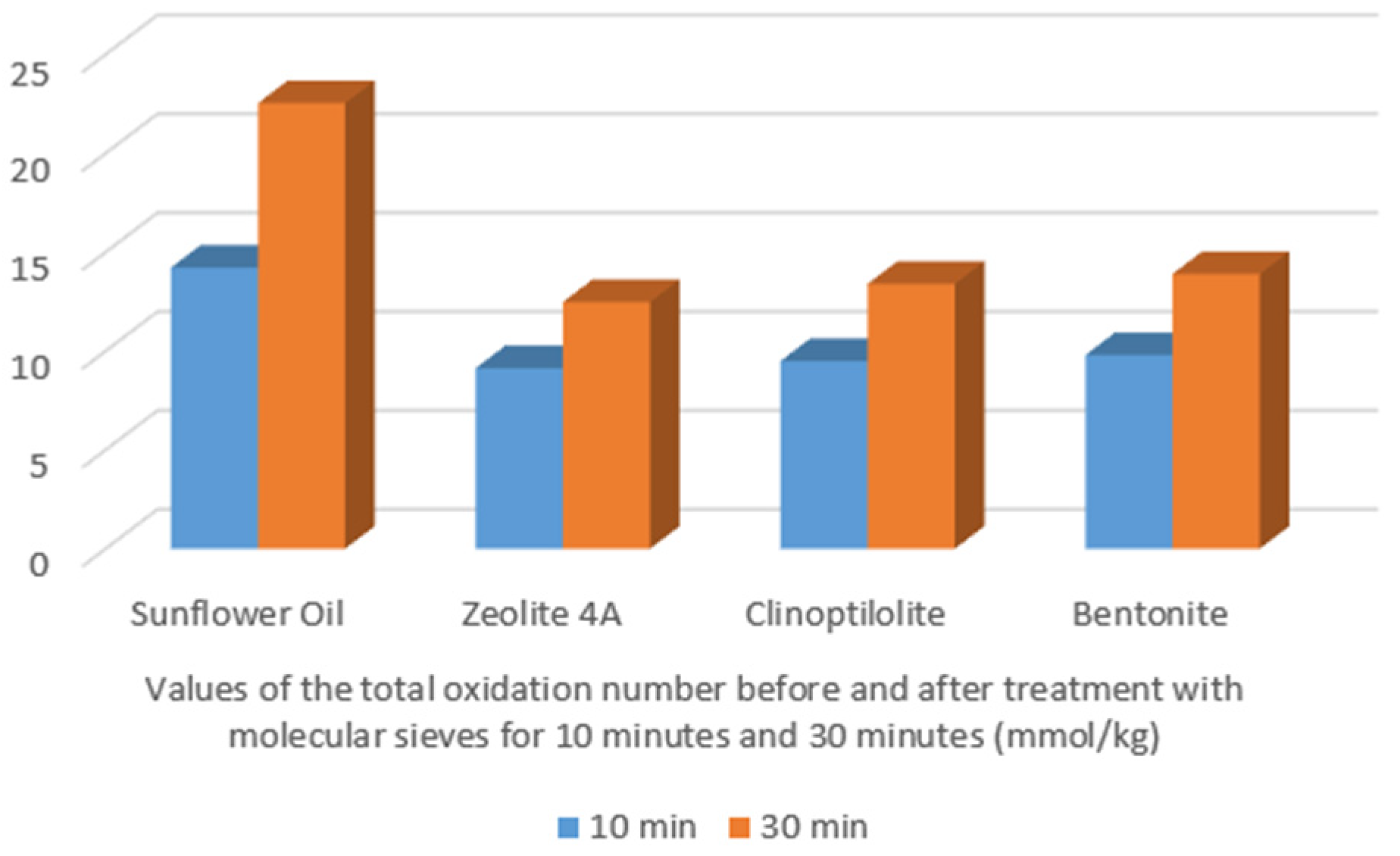
| Time (min) | Temperature (°C) | Peroxide Value (PV) | Anisidine Value (AV) | Totox Value (TV) |
|---|---|---|---|---|
| Sunflower Oil | - | 0.02 | 3.3 | 3.34 |
| 10 | 110 | 0.02 | 3.5 | 3.54 |
| 10 | 130 | 0.02 | 4.0 | 4.04 |
| 10 | 150 | 0.50 | 4.2 | 5.20 |
| 10 | 170 | 0.72 | 7.2 | 8.64 |
| 10 | 190 | 0.86 | 12.5 | 14.22 |
| 30 | 110 | 0.10 | 5.1 | 5.30 |
| 30 | 130 | 0.10 | 6.0 | 6.20 |
| 30 | 150 | 0.70 | 7.2 | 8.60 |
| 30 | 170 | 0.95 | 10.2 | 12.10 |
| 30 | 190 | 1.22 | 20.1 | 22.54 |
| Time (min) | Temperature (°C) | Peroxide Value (PV) | Anisidine Value (AV) | Totox Value (TV) |
|---|---|---|---|---|
| Sunflower oil | - | 0.02 | 3.3 | 3.34 |
| 10 | 110 | 0.02 | 3.0 | 3.04 |
| 10 | 130 | 0.02 | 3.0 | 3.04 |
| 10 | 150 | 0.40 | 3.2 | 4.00 |
| 10 | 170 | 0.60 | 4.7 | 5.90 |
| 10 | 190 | 0.72 | 7.7 | 9.14 |
| 30 | 110 | 0.05 | 3.5 | 3.60 |
| 30 | 130 | 0.05 | 3.7 | 3.80 |
| 30 | 150 | 0.50 | 4.0 | 5.00 |
| 30 | 170 | 0.35 | 4.7 | 5.40 |
| 30 | 190 | 0.74 | 11.0 | 12.48 |
| Time (min) | Temperature (°C) | Peroxide Value (PV) | Anisidine Value (AV) | Totox Value (TV) |
|---|---|---|---|---|
| Sunflower oil | - | 0.02 | 3.3 | 3.34 |
| 10 | 110 | 0.02 | 3.2 | 3.24 |
| 10 | 130 | 0.02 | 3.2 | 3.24 |
| 10 | 150 | 0.32 | 3.5 | 4.14 |
| 10 | 170 | 0.50 | 5.0 | 6.00 |
| 10 | 190 | 0.70 | 8.1 | 9.50 |
| 30 | 110 | 0.05 | 3.5 | 3.60 |
| 30 | 130 | 0.05 | 3.7 | 4.10 |
| 30 | 150 | 0.50 | 4.0 | 5.20 |
| 30 | 170 | 0.35 | 4.7 | 6.20 |
| 30 | 190 | 0.74 | 11.0 | 13.40 |
| Time (min) | Temperature (°C) | Peroxide Value (PV) | Anisidine Value (AV) | Totox Value (TV) |
|---|---|---|---|---|
| Sunflower oil | - | 0.02 | 3.3 | 3.34 |
| 10 | 110 | 0.02 | 3.5 | 3.54 |
| 10 | 130 | 0.02 | 2.5 | 2.54 |
| 10 | 150 | 0.32 | 2.6 | 3.24 |
| 10 | 170 | 0.50 | 5.2 | 3.60 |
| 10 | 190 | 0.70 | 8.4 | 9.80 |
| 30 | 110 | 0.05 | 3.7 | 3.80 |
| 30 | 130 | 0.05 | 4.5 | 4.60 |
| 30 | 150 | 0.33 | 4.6 | 5.26 |
| 30 | 170 | 0.45 | 5.8 | 6.70 |
| 30 | 190 | 0.70 | 12.5 | 13.90 |
| Totox Value (TV) | ||||
|---|---|---|---|---|
| Time (min) | Sunflower Oil | Zeolite 4A | Clinoptilolite | Bentonite |
| 10 | 110 | 0.02 | 3.2 | 3.24 |
| 30 | 190 | 0.74 | 11.0 | 13.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasiljević, L.; Dobrnjac, S.; Blagojević, S.; Aćimović, M. Assessment of the Oxidative State of Thermally Treated Sunflower Oil After Regeneration with Molecular Sieves. Eng. Proc. 2025, 99, 20. https://doi.org/10.3390/engproc2025099020
Vasiljević L, Dobrnjac S, Blagojević S, Aćimović M. Assessment of the Oxidative State of Thermally Treated Sunflower Oil After Regeneration with Molecular Sieves. Engineering Proceedings. 2025; 99(1):20. https://doi.org/10.3390/engproc2025099020
Chicago/Turabian StyleVasiljević, Ljubica, Sanja Dobrnjac, Stevan Blagojević, and Milenko Aćimović. 2025. "Assessment of the Oxidative State of Thermally Treated Sunflower Oil After Regeneration with Molecular Sieves" Engineering Proceedings 99, no. 1: 20. https://doi.org/10.3390/engproc2025099020
APA StyleVasiljević, L., Dobrnjac, S., Blagojević, S., & Aćimović, M. (2025). Assessment of the Oxidative State of Thermally Treated Sunflower Oil After Regeneration with Molecular Sieves. Engineering Proceedings, 99(1), 20. https://doi.org/10.3390/engproc2025099020





