Ion Exchange of Na+ Ions with H+ Ions on ZSM-5 Zeolite Using Acetic Acid †
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grzybek, G.; Greluk, M.; Patulski, P.; Stelmachowski, P.; Tarach, K.; Slowik, G.; Rotko, M.; Valencia, S.; Rey, F.; Góra-Marek, K. Adjustment of the ZSM-5 zeolite support towards the efficient hydrogen production by ethanol steam reforming on cobalt catalysts. Chem. Eng. J. 2023, 467, 143239. [Google Scholar] [CrossRef]
- Wang, S.; He, Y.; Jiao, W.; Wang, J.; Fan, W. Recent experimental and theoretical studies on Al siting/acid site in zeolite framework. Curr. Opin. Chem. Eng. 2019, 23, 146–154. [Google Scholar] [CrossRef]
- Yue, B.; Liu, S.; Chai, Y.; Wu, G.; Guan, N.; Li, L. Zeolites for separation: Fundamental and application. J. Energy Chem. 2022, 71, 288–303. [Google Scholar] [CrossRef]
- Rakanović, M.; Vukojević, A.; Savanović, M.M.; Armaković, S.; Pelemiš, S.; Živić, F.; Sladojević, S.; Armaković, S.J. Zeolites as adsorbents and photocatalysts for removal of dyes from the aqueous environment. Molecules 2022, 27, 6582. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Botella, E.; Valencia, S.; Rey, F. Zeolites in adsorption processes: State of the art and future prospects. Chem. Rev. 2022, 122, 17647–17695. [Google Scholar] [CrossRef] [PubMed]
- Corma, A. State of the art and future challenges of zeolites as catalysts. J. Catal. 2003, 216, 298–312. [Google Scholar] [CrossRef]
- Li, Y.; Yu, J. Emerging applications of zeolites in catalysis, separation and host–guest assembly. Nat. Rev. Mater. 2021, 6, 1156–1174. [Google Scholar] [CrossRef]
- Ulfiati, R. Catalytic performance of ZSM-5 zeolite in heavy hydrocarbon catalytic cracking: A review. Sci. Contrib. Oil Gas 2020, 42, 29–34. [Google Scholar] [CrossRef]
- Nuhma, M.J.; Alias, H.; Tahir, M.; Jazie, A.A. Microalgae biomass conversion into biofuel using modified HZSM-5 zeolite catalyst: A review. Mater. Today Proc. 2021, 42, 2308–2313. [Google Scholar] [CrossRef]
- Kumar, N.D.; Swaminathan, M. Review on Hierarchically Porous BEA and ZSM-5 Zeolites and Its Industrial Catalytic Applications. ES Mater. Manuf. 2024, 24, 1151. [Google Scholar] [CrossRef]
- Hernández, M.A.; Abbaspourrad, A.; Petranovskii, V.; Rojas, F.; Portillo, R.; Salgado, M.A.; Hernández, G.; Velazco, M.A.; Ayala, E.; Quiroz, K.F. Estimation of Nanoporosity of ZSM-5 Zeolites as Hierarchical Materials. In Zeolites and Their Applications, 1st ed.; Rashed, M.N., Palanisamy, P.N., Eds.; IntechOpen: London, UK, 2018; pp. 73–75. [Google Scholar]
- Murphy, B.; Davis, M.E.; Xu, B. The effect of adsorbed molecule gas-phase deprotonation enthalpy on ion exchange in sodium exchanged zeolites: An in situ FTIR investigation. Top. Catal. 2015, 58, 393–404. [Google Scholar] [CrossRef]
- Shirazi, L.; Jamshidi, E.; Ghasemi, M.R. The effect of Si/Al ratio of ZSM-5 zeolite on its morphology, acidity and crystal size. Cryst. Res. Technol. 2008, 43, 1300–1306. [Google Scholar] [CrossRef]
- Widayat, W.; Annisa, A.N. Synthesis and characterization of ZSM-5 catalyst at different temperatures. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Bandung, West Java, Indonesia, 17 July 2017. [Google Scholar]
- Armaroli, T.; Simon, L.J.; Digne, M.; Montanari, T.; Bevilacqua, M.; Valtchev, V.; Patarin, J.; Busca, G. Effects of crystal size and Si/Al ration on the surface properties of H-ZSM-5 zeolites. Appl. Catal. A Gen. 2006, 306, 78–84. [Google Scholar] [CrossRef]
- Baerlocher, C.; McCusker, L.B.; Olson, D.H. MFI. In Atlas of Zeolite Framework Type, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 212–218. [Google Scholar]
- Wu, W.; Weitz, E. Modification of acid sites in ZSM-5 by ion-exchange: An in-situ FTIR study. Appl. Surf. Sci. 2014, 316, 405–415. [Google Scholar] [CrossRef]
- Silva, V.J.; Crispim, A.C.; Queiroz, M.B.; Menezes, R.R.; Laborde, M.; Rodrigues, M.G.F. Characterization structural and morphology ZSM-5 zeolite by hydrothermal synthesis. In Proceeding of the Seventh International Latin American Conference on Powder Technology, Atibaia, Brazil, 8–10 November 2009. [Google Scholar]
- Shen, Y.; Qin, Z.; Asahina, S.; Asano, N.; Zhang, G.; Qian, S.; Yanhang, M.; Yan, Z.; Liu, X.; Mintova, S. The inner heterogeneity of ZSM-5 zeolite crystals. J. Mater. Chem. A 2021, 9, 4203. [Google Scholar] [CrossRef]
- YS/T 575.19-2021; Methods for Chemical Analysis of Bauxite—Part 19: Determination of Loss on Ignition—Gravimetric Method. China Nonferrous Metals Industry Standardization Institute: Beijing, China, 2021.
- ISO 11885:2007; Water Quality Determination of Selected Elements by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES). ISO: Geneva, Switzerland, 2007.
- ASTM D5758-01; Standard Test Method for Determination of Relative Crystallinity of Zeolite ZSM-5 by X-Ray Diffraction. ASTM International: West Conshohocken, PA, USA, 2021.
- Škrba, M.; Obrenović, Z.; Došić, A.; Gligorić, M.; Đurić, B.; Savić, I. Ion exchange of sodium with hydrochloric acid in ZSM-5 zeolite. Mater. Prot. 2021, 62, 155–165. [Google Scholar] [CrossRef]
- Danaher, P.J.; Medino, C.; Shevchuk, H.; Zhang, E.M. Testing the Stability of Cation Exchanged Zeolite ZSM-5 in Hot Liquid Water. Available online: https://digital.wpi.edu/downloads/gf06g4038 (accessed on 8 March 2025).

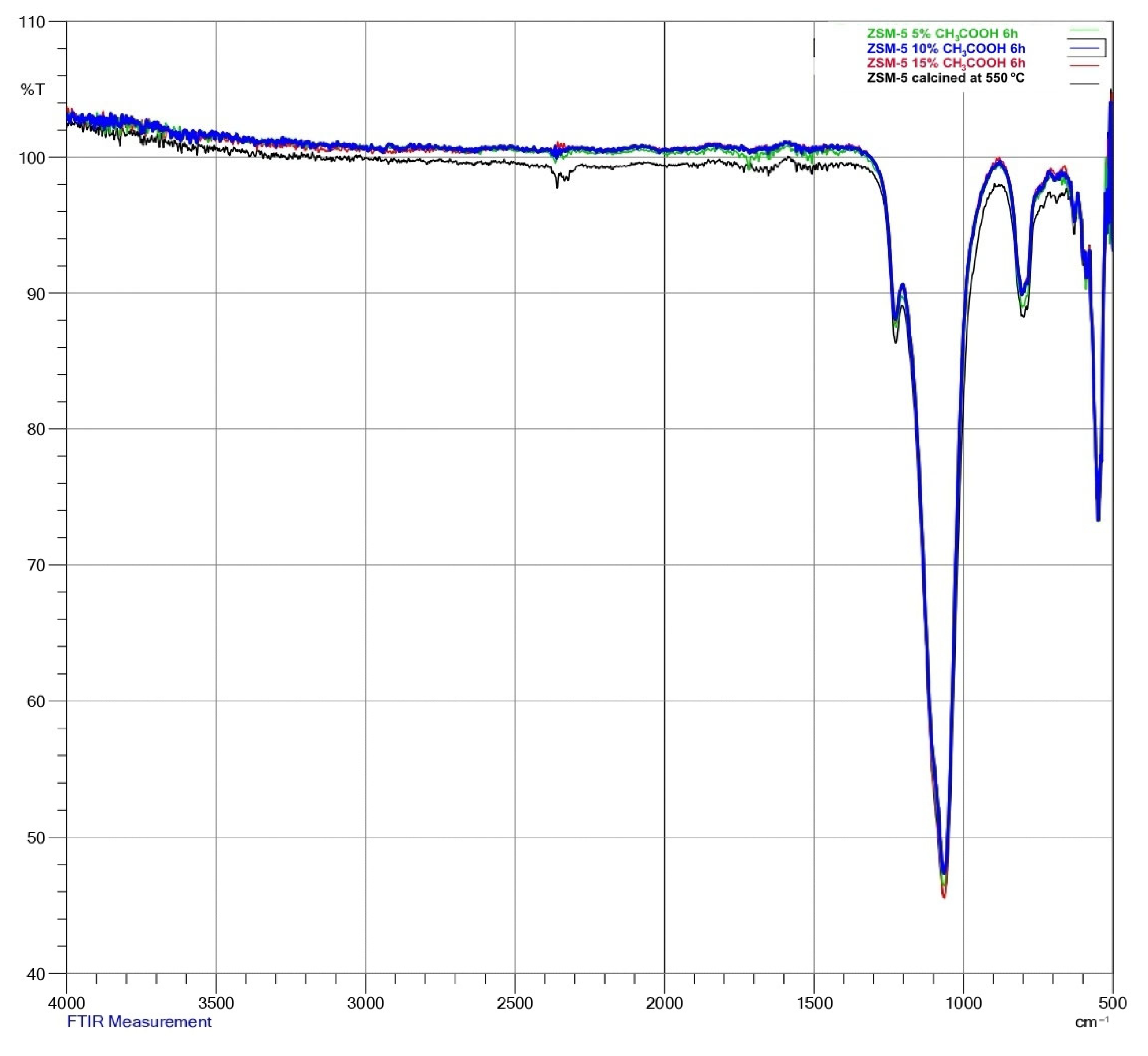
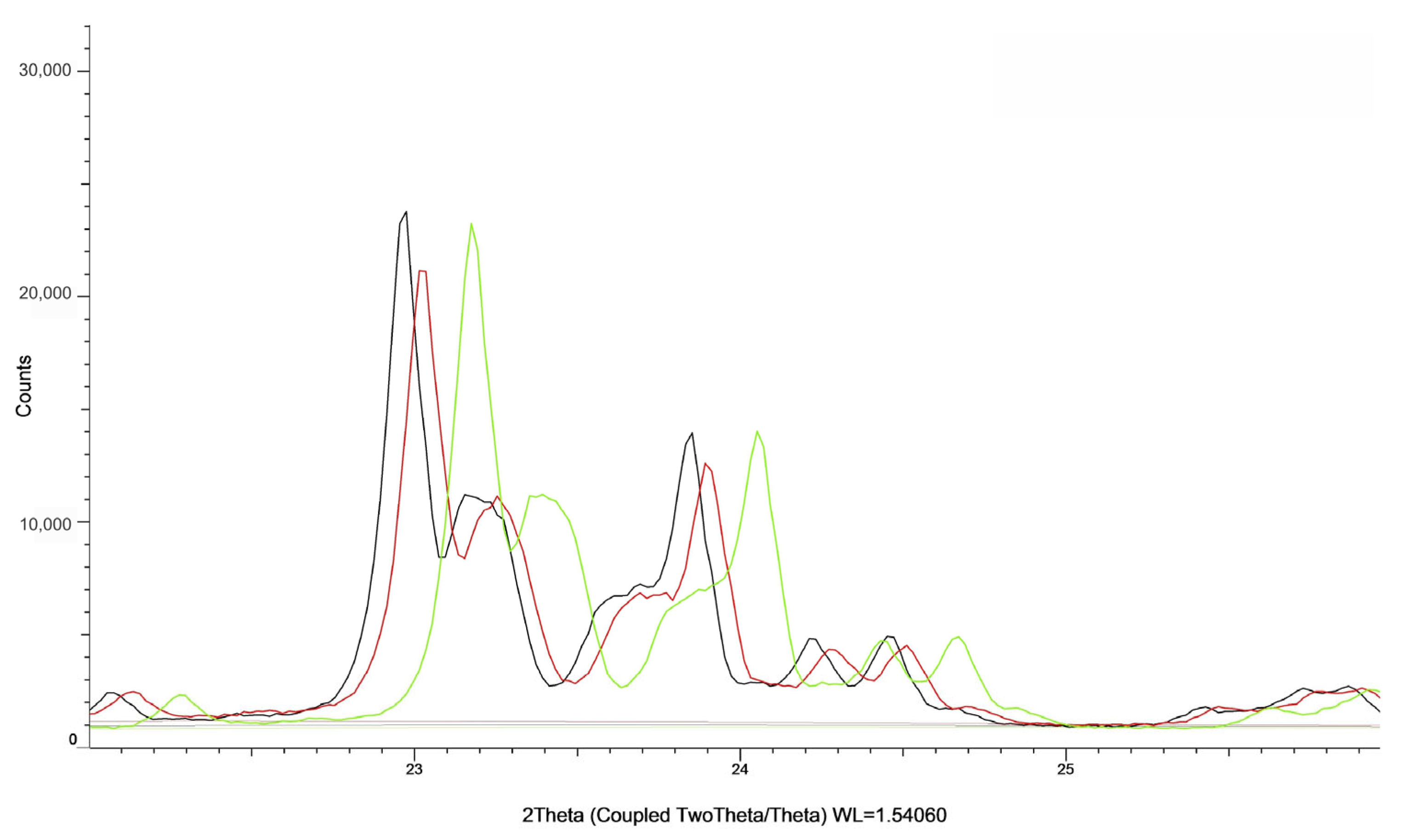
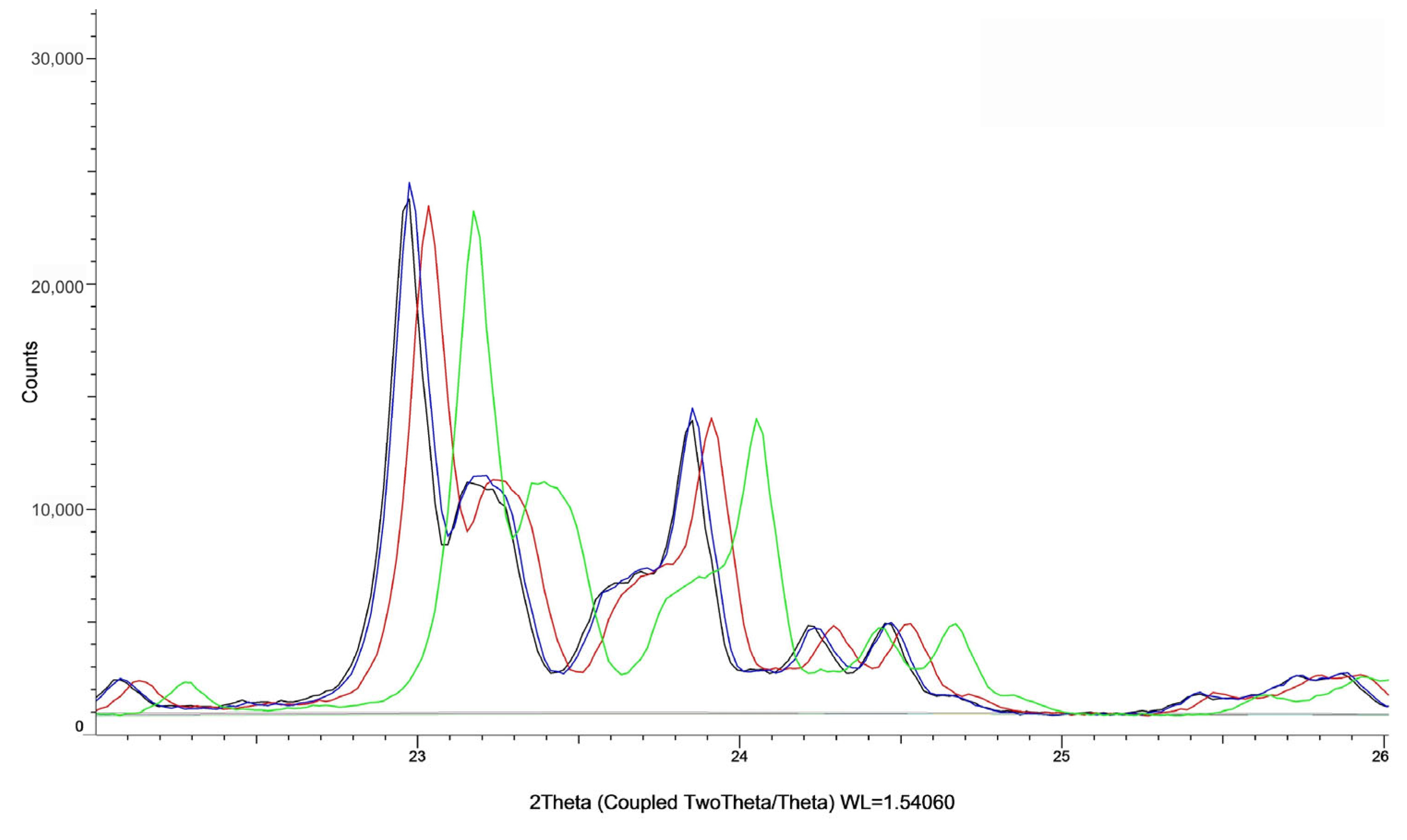
| Sample | Ion Exchange Time [h] | Acid Concentration [%] | Loss on Ignition [%] | %Na | %Al2O3 |
|---|---|---|---|---|---|
| calcined ZSM-5 | / | / | 4.01 | 1.08 | 0.18 |
| ZSM-5 5% CH3COOH 6 h | 6 | 5 | 1.64 | 0.13 | 0.17 |
| ZSM-5 10% CH3COOH 1 h | 1 | 10 | 1.67 | 0.044 | 0.17 |
| ZSM-5 10% CH3COOH 3 h | 3 | 10 | 4.69 | 0.048 | 0.16 |
| ZSM-5 10% CH3COOH 6 h | 6 | 10 | 1.57 | 0.13 | 0.16 |
| ZSM-5 15% CH3COOH 1 h | 1 | 15 | 3.26 | 0.041 | 0.16 |
| ZSM-5 15% CH3COOH 3 h | 3 | 15 | 2.89 | 0.046 | 0.15 |
| ZSM-5 15% CH3COOH 6 h | 6 | 15 | 0.04 | 0.17 | 0.15 |
| Sample | Degree of Crystallinity [%] |
|---|---|
| calcined ZSM-5 | 94.83 |
| ZSM-5 5% CH3COOH 6 h | 87.11 |
| ZSM-5 15% CH3COOH 1 h | 94.74 |
| ZSM-5 15% CH3COOH 3 h | 95.56 |
| ZSM-5 15% CH3COOH 6 h | 92.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Došić, A.; Obrenović, M.; Obrenović, Z.; Vuković, J.; Savić, I.M. Ion Exchange of Na+ Ions with H+ Ions on ZSM-5 Zeolite Using Acetic Acid. Eng. Proc. 2025, 99, 17. https://doi.org/10.3390/engproc2025099017
Došić A, Obrenović M, Obrenović Z, Vuković J, Savić IM. Ion Exchange of Na+ Ions with H+ Ions on ZSM-5 Zeolite Using Acetic Acid. Engineering Proceedings. 2025; 99(1):17. https://doi.org/10.3390/engproc2025099017
Chicago/Turabian StyleDošić, Aleksandar, Milomirka Obrenović, Zoran Obrenović, Jelena Vuković, and Ivan M. Savić. 2025. "Ion Exchange of Na+ Ions with H+ Ions on ZSM-5 Zeolite Using Acetic Acid" Engineering Proceedings 99, no. 1: 17. https://doi.org/10.3390/engproc2025099017
APA StyleDošić, A., Obrenović, M., Obrenović, Z., Vuković, J., & Savić, I. M. (2025). Ion Exchange of Na+ Ions with H+ Ions on ZSM-5 Zeolite Using Acetic Acid. Engineering Proceedings, 99(1), 17. https://doi.org/10.3390/engproc2025099017







