Fabrication and Characterization of Tungsten-Modified TiO2 as a Photo-Anode in a Dye-Sensitized Solar Cell †
Abstract
1. Introduction
2. Experiment
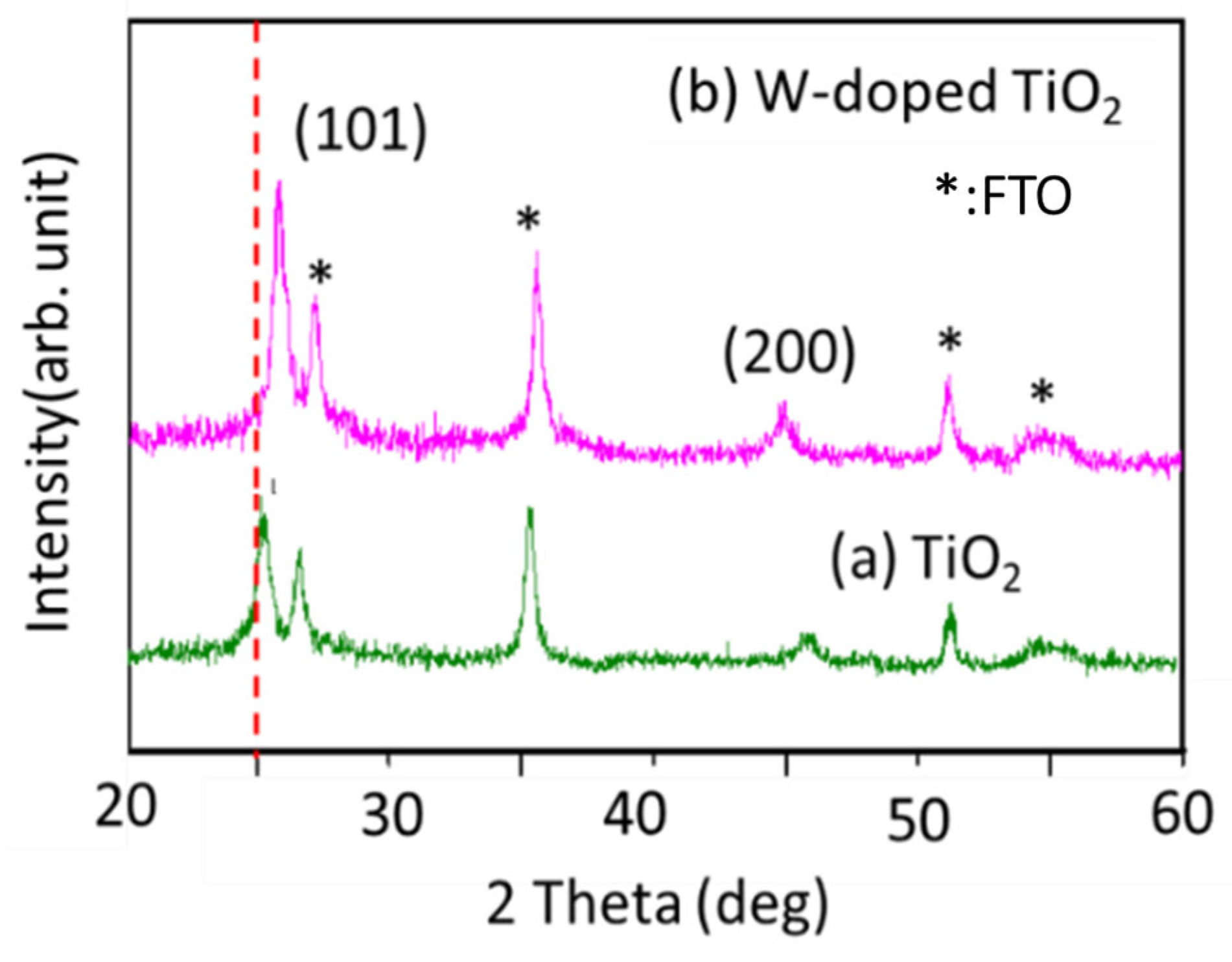
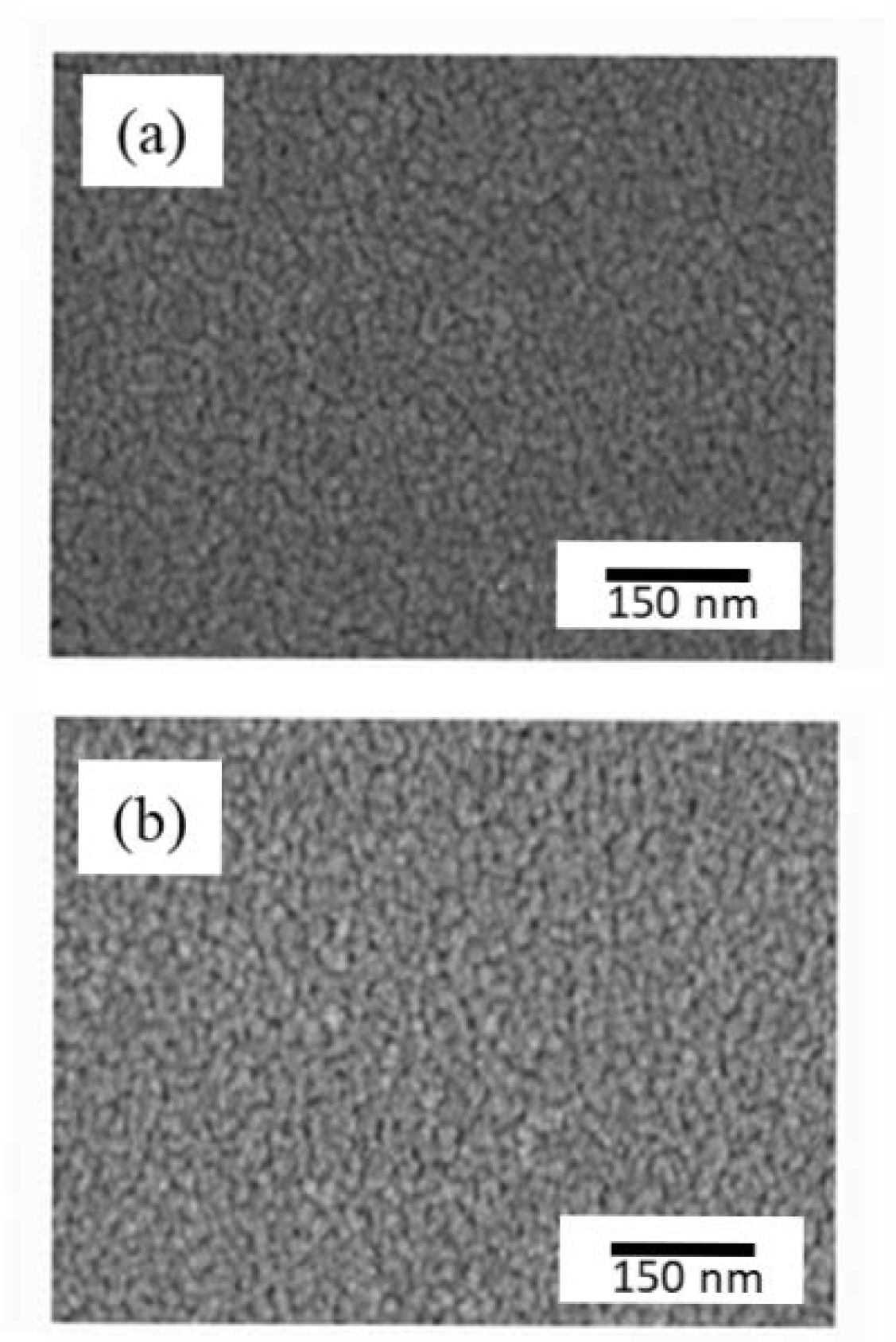
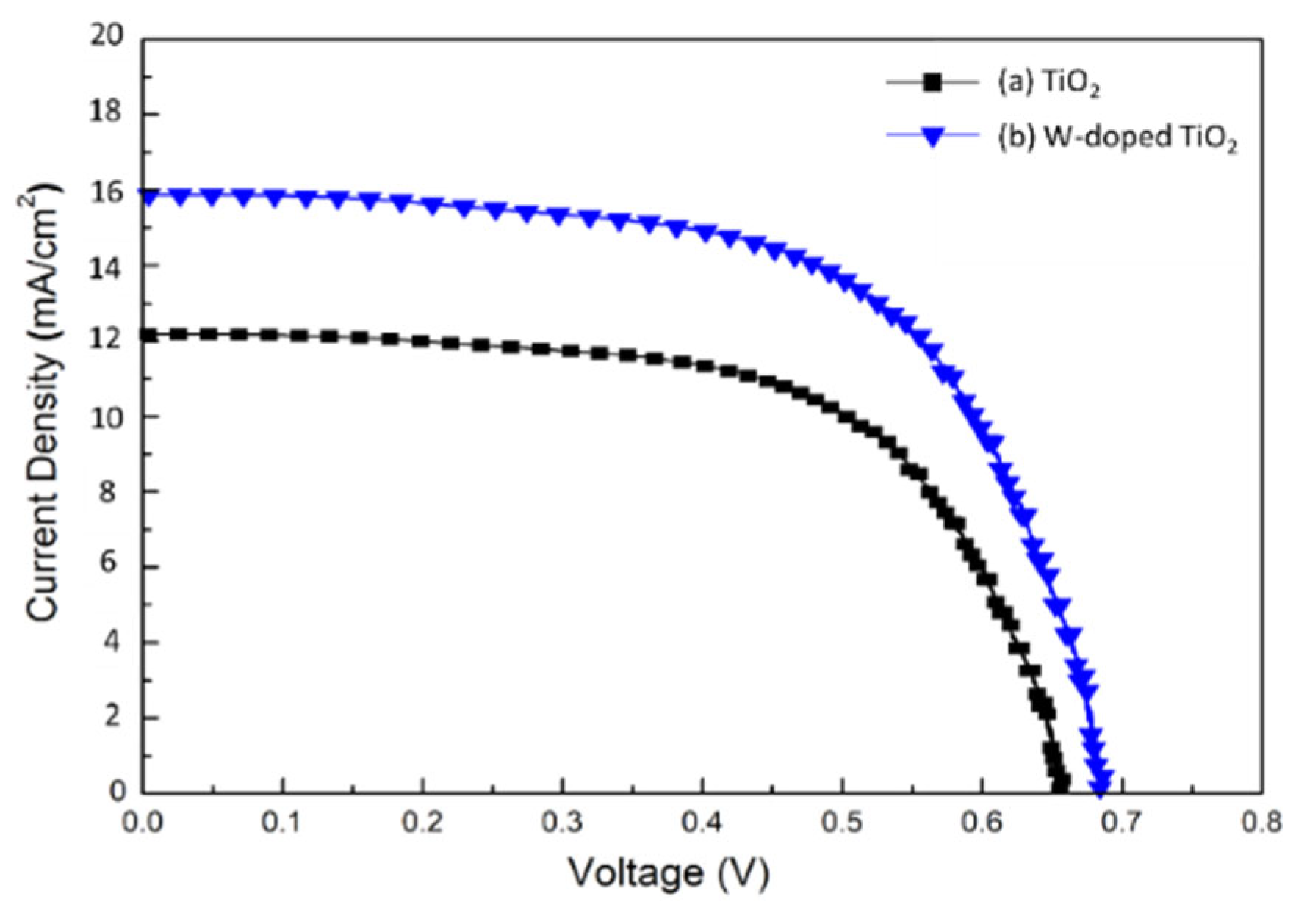
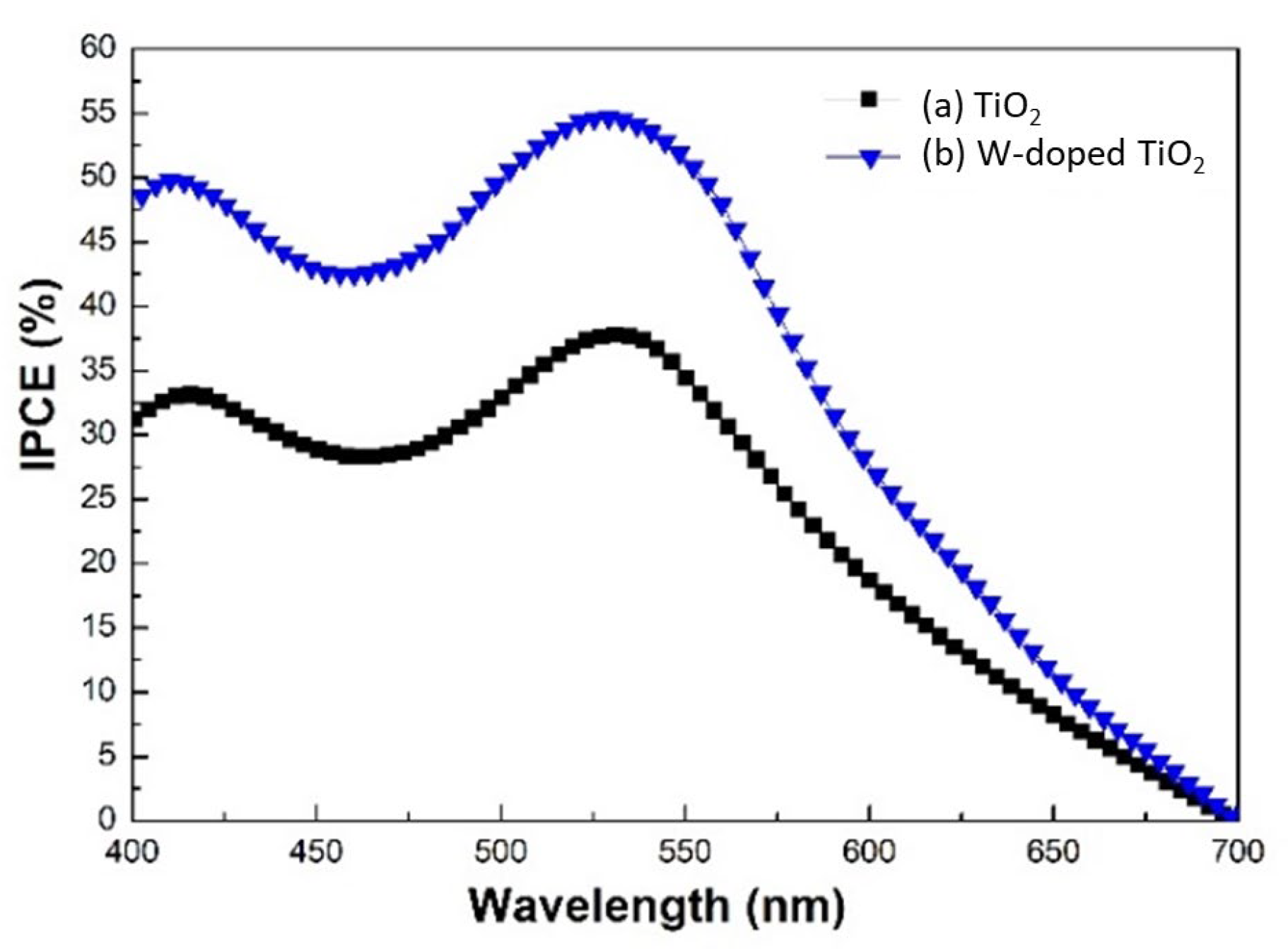
3. Result and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agrawal, A.; Siddiqui, S.A.; Soni, A.; Khandelwal, K.; Sharma, G.D. Performance analysis of TiO2 based dye sensitized solar cell prepared by screen printing and doctor blade deposition techniques. Sol. Energy 2021, 226, 9–19. [Google Scholar] [CrossRef]
- Abdullaev, S.S.; Breesam, Y.F.; AlZubaidi, A.A.H.; Tripathi, A.K.; Kareem, A.K.; Kuznetsov, S.V.; Alawsi, T.; Zabibah, R.S. ZnO@ZnCo2O4 core-shell: A novel high electrocatalytic nanostructure to replace platinum as the counter electrode in dye-sensitized solar cells. Mater. Sci. Semicond. Process. 2023, 165, 107709. [Google Scholar] [CrossRef]
- Patil, S.S.; Mane, R.M.; Khot, K.V.; Mali, S.S.; Hong, C.K.; Bhosale, P.N. Surfactant assisted approach to development of efficient WO3 photoanode for natural dye sensitized solar cell. Sol. Energy 2021, 220, 371–383. [Google Scholar] [CrossRef]
- Abrari, M.; Ghanaatshoar, M.; Davarani, S.S.H.; Moazami, H.R.; Kazeminezhad, I. Synthesis of SnO2 nanoparticles by electro oxidation of tin in quaternary ammonium salt for application in dye-sensitized solar cells. Appl. Phys. A 2017, 123, 326–329. [Google Scholar] [CrossRef]
- Prabakaran, S.; Nisha, K.D.; Harish, S.; Archana, J.; Navaneethan, M.; Ponnusamy, S.; Muthamizhchelvan, C.; Ikeda, H.; Hayakawa, Y. Synergistic effect and enhanced electrical properties of TiO2/SnO2/ZnO nanostructures as electron extraction layer for solar cell application. Appl. Surf. Sci. 2019, 498, 143702. [Google Scholar] [CrossRef]
- Vibavakumar, S.; Nish, K.D.; Manikandan, V.S.; Archana, J.; Navaneethan, M.; Harish, S. Enhanced photo conversion efficiency of Nb2O5/TiO2 bilayer photoanode for Dye-Sensitized Solar Cells. Optical Mater. 2023, 140, 113828–113835. [Google Scholar] [CrossRef]
- Han, Q.; Wu, C.; Jiao, H.; Xu, R.; Wang, Y.; Xie, J.; Guo, Q.; Tang, J. Rational design of high-concentration Ti3+ in porous carbon-doped TiO2 nanosheets for efficient photocatalytic ammonia synthesis. Adv. Mater. 2021, 33, 2008180. [Google Scholar] [CrossRef]
- Guo, Q.; Wu, J.; Yang, Y.; Liu, X.; Sun, W.; Wei, Y.; Lan, Z.; Lin, J.; Huang, M.; Chen, H.; et al. Low-temperature processed rare-earth doped brookite TiO2 scaffold for UV stable, hysteresis-free and high-performance perovskite solar cells. Nano Energy 2020, 77, 105183. [Google Scholar] [CrossRef]
- Liu, G.; Huang, M.; Zhang, Z.; Xi, B.; Li, H.; Xiong, S. Robust S-doped TiO2@N,S-codoped carbon nanotube arrays as free-binder anodes for efficient sodium storage. J. Energy Chem. 2021, 53, 175–184. [Google Scholar] [CrossRef]
- Liao, Y.-H.; Chang, Y.-H.; Lin, T.-H.; Chan, S.-H.; Lee, K.-M.; Hsu, K.-H.; Hsu, J.-F.; Wu, M.-C. Boosting the power conversion efficiency of perovskite solar cells based on Sn doped TiO2 electron extraction layer via modification the TiO2 phase junction. Sol. Energy 2020, 205, 390–398. [Google Scholar] [CrossRef]
- Katta, V.S.; Chappidi, V.R.; Raavi, S.S.K. Samarium-doped TiO2 photoanodes for the molecular devices for solar energy conversion. In Proceedings of the SPIE/COS Photonics Asia, Nantong, China, 10–20 October 2021. [Google Scholar] [CrossRef]
- Katta, V.S.; Velpandian, M.; Chappidi, V.R.; Ahmed, M.S.; Kumar, A.; Asthana, S.; Meduri, P.; Raavi, S.S.K. Er3+ doped titania photoanode for enhanced performance of photo-electrochemical water splitting devices. Mater. Lett. 2021, 302, 130297. [Google Scholar] [CrossRef]
- Tang, X.; Xue, Q.; Qi, X.; Cheng, C.; Yang, M.; Yang, T.; Chen, F.; Qiu, F.; Quan, X. DFT and experimental study on visible-light driven photocatalysis of rare-earth-doped TiO2. Vacuum 2022, 200, 110972. [Google Scholar] [CrossRef]
- Kumaravel, V.; Rhatigan, S.; Mathew, S.; Bartlett, J.; Nolan, M.; Hinder, S.J.; Sharma, P.K.; Singh, A.; Byrne, J.A.; Harrison, J.; et al. Indium-doped TiO2 photocatalysts with high-temperature anatase stability. J. Phys. Chem. C 2019, 123, 21083–21096. [Google Scholar] [CrossRef]
- Kong, X.; Peng, Z.; Jiang, R.; Jia, P.; Feng, J.; Yang, P.; Chi, Q.; Ye, W.; Xu, F.; Gao, P. Nanolayered heterostructures of N-doped TiO2 and N-doped carbon for hydrogen evolution. ACS Appl. Nano Mater. 2020, 3, 1373–1381. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Q.; Li, S.; Wang, Y.; Ye, C.; Ruterana, P.; Wang, H. An efficient photoanode consisting of TiO2 nanoparticle-filled TiO2 nanotube arrays for dye-sensitized solar cells. J. Power Sources 2014, 268, 941–949. [Google Scholar] [CrossRef]
- Su, C.-Y.; Wang, L.-C.; Liu, W.-S.; Wang, C.-C.; Perng, T.-P. Photocatalysis and hydrogen evolution of Al- and Zn-doped TiO2 nanotubes fabricated by atomic layer deposition. ACS Appl. Mater. Interfaces 2018, 10, 33287–33295. [Google Scholar] [CrossRef]
- Raguram, T.; Rajni, K.S. Synthesis and characterization of Cu-doped TiO2 nanoparticles for DSSC and photocatalytic applications. Int. J. Hydrogen Energy 2022, 47, 4674–4689. [Google Scholar] [CrossRef]
- Subalakshmi, K.; Senthilselvan, J. Effect of fluorine-doped TiO2 photoanode on electron transport, recombination dynamics and improved DSSC efficiency. Sol. Energy 2018, 171, 914–928. [Google Scholar] [CrossRef]
- Yun, S.; Zhou, X.; Zhang, Y.; Wang, C.; Hou, Y. Tantalum-based bimetallic oxides deposited on spherical carbon of biological origin for use as counter electrodes in dye sensitized solar cells. Electrochim. Acta 2019, 309, 371–381. [Google Scholar] [CrossRef]
- Grbić, B.; Radić, N.; Stojadinović, S.; Vasilić, R.; Mitrović, Z.D.; Šaponjić, Z.; Stefanov, P. TiO2/WO3 photocatalytic composite coatings prepared by spray pyrolysis. Surf. Coating. Technol. 2014, 258, 763–771. [Google Scholar] [CrossRef]
- Aboud, A.A.; Mukherjee, A.; Revaprasadu, N.; Mohamed, A.N. The effect of Cu-doping on CdS thin films deposited by the spray pyrolysis technique. J. Mater. Res. Technol. 2019, 8, 2021–2030. [Google Scholar] [CrossRef]
- Dave, D.P.; Chauhan, K.V. Synthesis of visible spectrum-active TiO2 thin film induced by RF magnetron sputtering. Mater. Today Proc. 2022, 62, 4254–4259. [Google Scholar] [CrossRef]
- Daviosdottir, S.; Shabadi, R.; Galca, A.C.; Andersend, I.H.; Dirscherl, K.; Ambata, R. Investigation of DC magnetron-sputtered TiO2 coatings: Effect of coating thickness, structure, and morphology on photocatalytic activity. Appl. Surf. Sci. 2014, 313, 677–686. [Google Scholar] [CrossRef]
- Miszczak, S.; Pietrzyk, B. Anatase–rutile transformation of TiO2 sol–gel coatings deposited on different substrates. Ceram. Int. 2015, 41, 7461–7465. [Google Scholar] [CrossRef]
- Azizpour, H.; Talebi, M.; Tichelaar, F.D.; Sotudeh-Gharebagh, R.; Guo, J.; van Ommen, J.R.; Mostoufi, N. Effective coating of titania nanoparticles with alumina via atomic layer deposition. Appl. Surf. Sci. 2017, 426, 480–496. [Google Scholar] [CrossRef]
- Miyata, T.; Tsukada, S.; Minami, T. Preparation of anatase TiO2 thin films by vacuum arc plasma evaporation. Thin Solid Films 2006, 491, 136–140. [Google Scholar] [CrossRef]
- Bhabu, K.A.; Devi, A.K.; Theerthagiri, J.; Madhavan, J.; Balu, T.; Rajasekaran, T.R. Tungsten doped titanium dioxide as a photoanode for dye sensitized solar cells. J. Mater. Sci. Mater. Electron. 2017, 28, 3428–3439. [Google Scholar] [CrossRef]
- Sathasivam, S.; Bhachu, D.; Lu, Y.; Chadwick, N.; Althabaiti, S.A.; Alyoubi, A.O.; Basahel, S.N.; Carmalt, C.J.; Parkin, I.P. Tungsten Doped TiO2 with Enhanced Photocatalytic and Optoelectrical Properties via Aerosol Assisted Chemical Vapor Deposition. Sci. Rep. 2015, 5, 10952. [Google Scholar] [CrossRef]
- Irie, H.; Watanabe, Y.; Hashimoto, K. Carbon-doped Anatase TiO2 Powders as a Visible-light Sensitive Photocatalyst. Chem. Lett. 2003, 32, 772–773. [Google Scholar] [CrossRef]
- Wategaonkar, S.B.; Parale, V.G.; Pawar, R.P.; Mali, S.S.; Hong, C.K.; Powar, R.R.; Moholkar, A.V.; Park, H.-H.; Sargar, B.M.; Mane, R.K. Structural, morphological, and optical studies of hydrothermally synthesized Nb-added TiO2 for DSSC application. Ceram. Int. 2021, 47, 25580–25592. [Google Scholar] [CrossRef]
| Samples | BET Surface Area (m2/g) | Amount of Dye in the Electrodes (10−8 mol/cm2) |
|---|---|---|
| TiO2 | 42 | 1.98 |
| Tungsten-modified TiO2 | 56 | 2.75 |
| Electrodes | Jsc (mA/cm2) | Voc (V) | Fill Factor (%) | Efficiency (η %) |
|---|---|---|---|---|
| TiO2 | 12.2 | 0.65 | 58.7 | 4.65 |
| W-doped TiO2 | 16.28 | 0.68 | 63.5 | 7.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kao, M.-C.; Weng, J.-H.; Chiang, C.-H.; Chen, K.-H.; Lin, D.-Y.; Kang, T.-K. Fabrication and Characterization of Tungsten-Modified TiO2 as a Photo-Anode in a Dye-Sensitized Solar Cell. Eng. Proc. 2025, 92, 76. https://doi.org/10.3390/engproc2025092076
Kao M-C, Weng J-H, Chiang C-H, Chen K-H, Lin D-Y, Kang T-K. Fabrication and Characterization of Tungsten-Modified TiO2 as a Photo-Anode in a Dye-Sensitized Solar Cell. Engineering Proceedings. 2025; 92(1):76. https://doi.org/10.3390/engproc2025092076
Chicago/Turabian StyleKao, Ming-Cheng, Jun-Hong Weng, Chih-Hung Chiang, Kai-Huang Chen, Der-Yuh Lin, and Tsung-Kuei Kang. 2025. "Fabrication and Characterization of Tungsten-Modified TiO2 as a Photo-Anode in a Dye-Sensitized Solar Cell" Engineering Proceedings 92, no. 1: 76. https://doi.org/10.3390/engproc2025092076
APA StyleKao, M.-C., Weng, J.-H., Chiang, C.-H., Chen, K.-H., Lin, D.-Y., & Kang, T.-K. (2025). Fabrication and Characterization of Tungsten-Modified TiO2 as a Photo-Anode in a Dye-Sensitized Solar Cell. Engineering Proceedings, 92(1), 76. https://doi.org/10.3390/engproc2025092076









