Preparation and Electrochemical Properties of Molybdenum Disulfide Nanomaterials †
Abstract
1. Introduction
2. Experiment
2.1. MoS2 Nanomaterials
2.2. Characterization
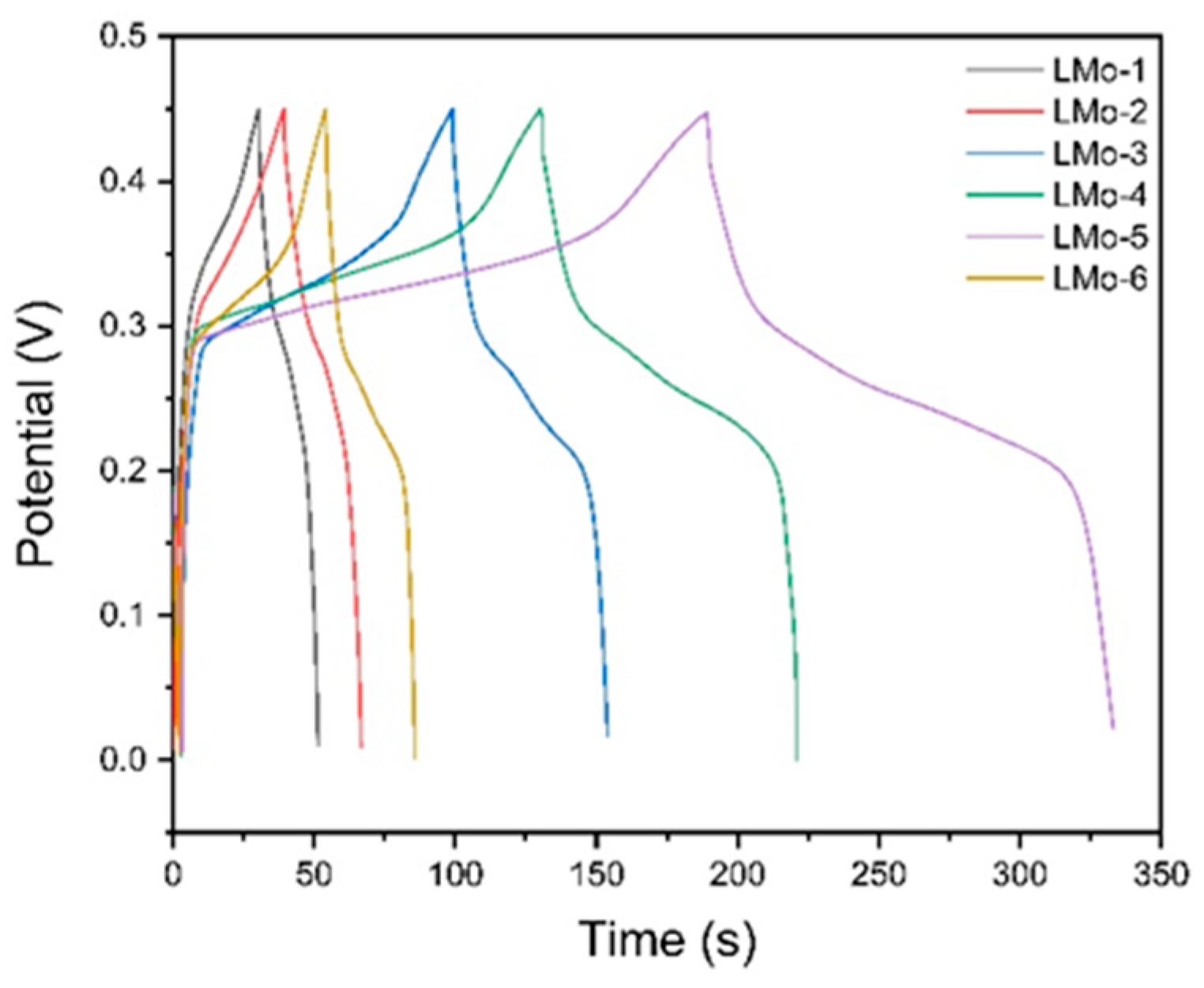
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.-X.; Seng, K.H.; Chou, S.-L.; Wang, J.-Z.; Guo, Z.; Wexler, D.; Liu, H.-K.; Dou, S.-X. Reversible sodium storage via conversion reaction of a MoS2–C composite. Chem. Commun. 2014, 50, 10730. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zeng, T.; Huang, D.; Yan, W.; Zhang, Y.; Wan, Q.; Yang, N. High-Energy-Density Supercapacitors from Dual Pseudocapacitive Nanoelectrodes, ACS Applied Energy Materials. ACS Appl. Energy Mater. 2020, 3, 10685. [Google Scholar] [CrossRef]
- Wardani, V.; Rohmawati, L.; Setyarsih, W.; Alfarisi, D.; Subhan, A.J. Analysis of Charging/Discharging Supercapacitor Active Carbon/rGO Based on Natural Materials. Phys. Conf. Ser. 2020, 1491, 012044. [Google Scholar] [CrossRef]
- Lu, X.; Zhai, T.; Zhang, X.; Shen, Y.; Yuan, L.; Hu, B.; Gong, L.; Chen, J.; Gao, Y.; Zhou, J.; et al. WO3–x@Au@MnO2 Core–Shell Nanowires on Carbon Fabric for High-Performance Flexible Supercapacitors. Adv. Mater. 2012, 24, 938. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.-Y.; Ferreira, J.M.F.; Sakka, Y.J. Hydrogen-Generation Materials for Portable Applications. Am. Ceram. Soc. 2008, 91, 3825. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors, Nature Materials. Nat. Mater. 2008, 7, 845. [Google Scholar] [CrossRef] [PubMed]
- Whittingham, M.S. The hydrated intercalation complexes of the layered disulfides. Mater. Res. Bull. 1974, 9, 1681. [Google Scholar] [CrossRef]
- Welkie, D.G.; Lagally, M.G. Analysis of surface structural defects by low energy electron diffraction. Thin Solid Film. 1982, 93, 219. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Q.; Li, C.; Piao, Y.; Hou, N.; Hu, K.J. Characterization of surface and subsurface defects induced by abrasive machining of optical crystals using grazing incidence X-ray diffraction and molecular dynamics. Adv. Res. 2022, 36, 51. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Han, D.; Wang, Y.; Wang, L.; Chen, X.; Qiao, X.; Yu, X. Synergy between nanozymes and natural enzymes on the hybrid MoS2 nanosheets/graphite microfiber for enhanced voltammetric determination of hydrogen peroxide. Microchim. Acta 2020, 187, 321. [Google Scholar] [CrossRef] [PubMed]
- Ju, L.; Hong, D.; Jin, X.; Liu, H.; Yang, X.; Nie, L.; Liu, Q.; Gao, Z.; Zhu, W.; Wang, Y.; et al. Preparation and Study of Photocatalytic Properties of (M(M=Pt, Ag and Au)-TiO2)@MoS2 Nanocomposites. Inorganics 2023, 11, 258. [Google Scholar] [CrossRef]
- Pei, J.; Yang, J.; Xu, R.; Zeng, Y.-H.; Myint, Y.W.; Zhang, S.; Zheng, J.-C.; Qin, Q.; Wang, X.; Jiang, W.; et al. Exciton and Trion Dynamics in Bilayer MoS2. Small 2015, 11, 6384. [Google Scholar] [CrossRef] [PubMed]
- Golovynskyi, S.; Irfan, I.; Bosi, M.; Seravalli, L.; Datsenko, O.I.; Golovynska, I.; Li, B.; Lin, D.; Qu, J. Exciton and trion in few-layer MoS2: Thickness- and temperature-dependent photoluminescence. Appl. Surf. Sci. 2020, 515, 146033. [Google Scholar] [CrossRef]
- Ma, L.; Huang, G.; Chen, W.; Wang, Z.; Ye, J.; Li, H.; Chen, D.; Lee, J.Y.J. Cationic surfactant-assisted hydrothermal synthesis of few-layer molybdenum disulfide/graphene composites: Microstructure and electrochemical lithium storage. Power Sources 2014, 264, 262. [Google Scholar] [CrossRef]
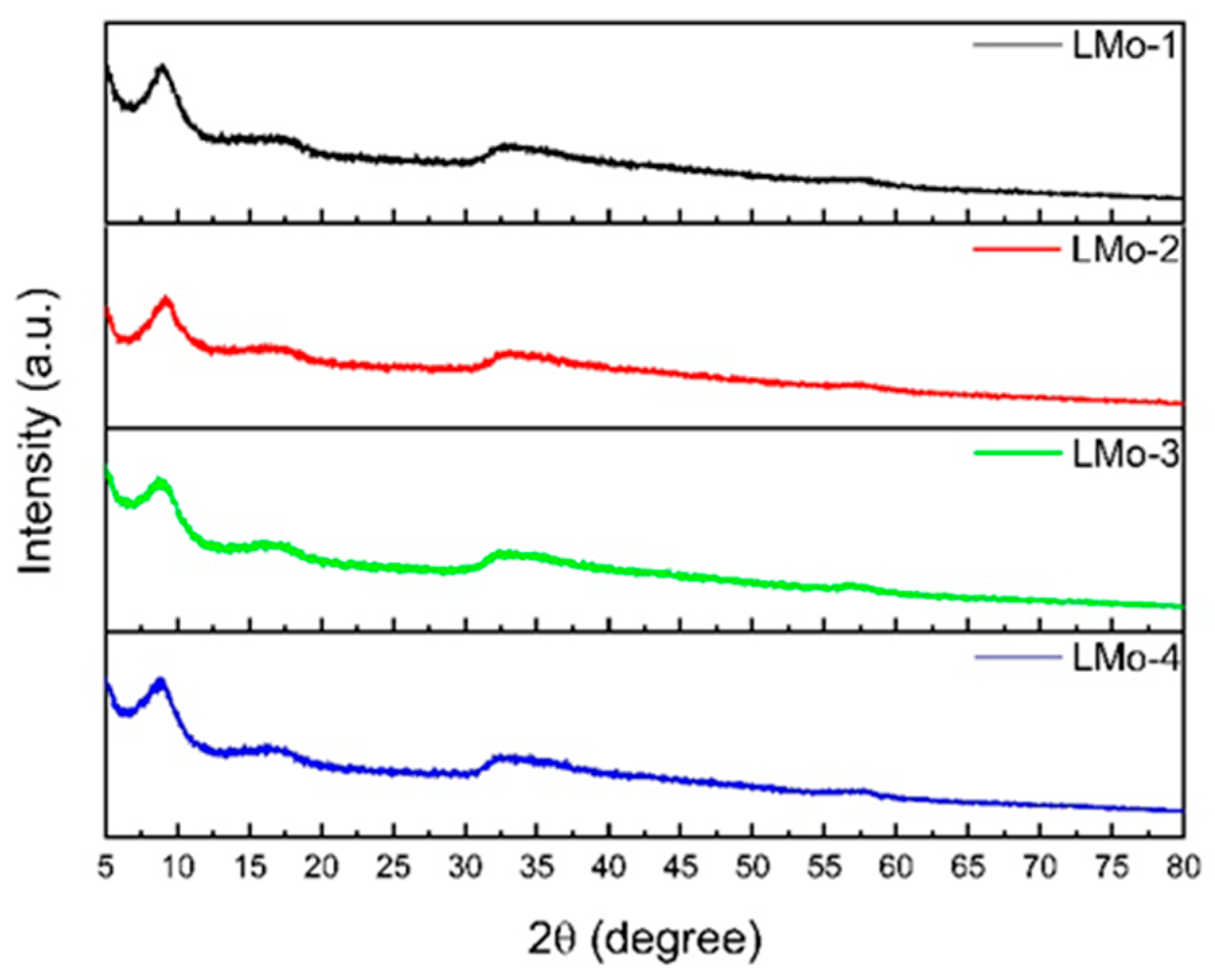
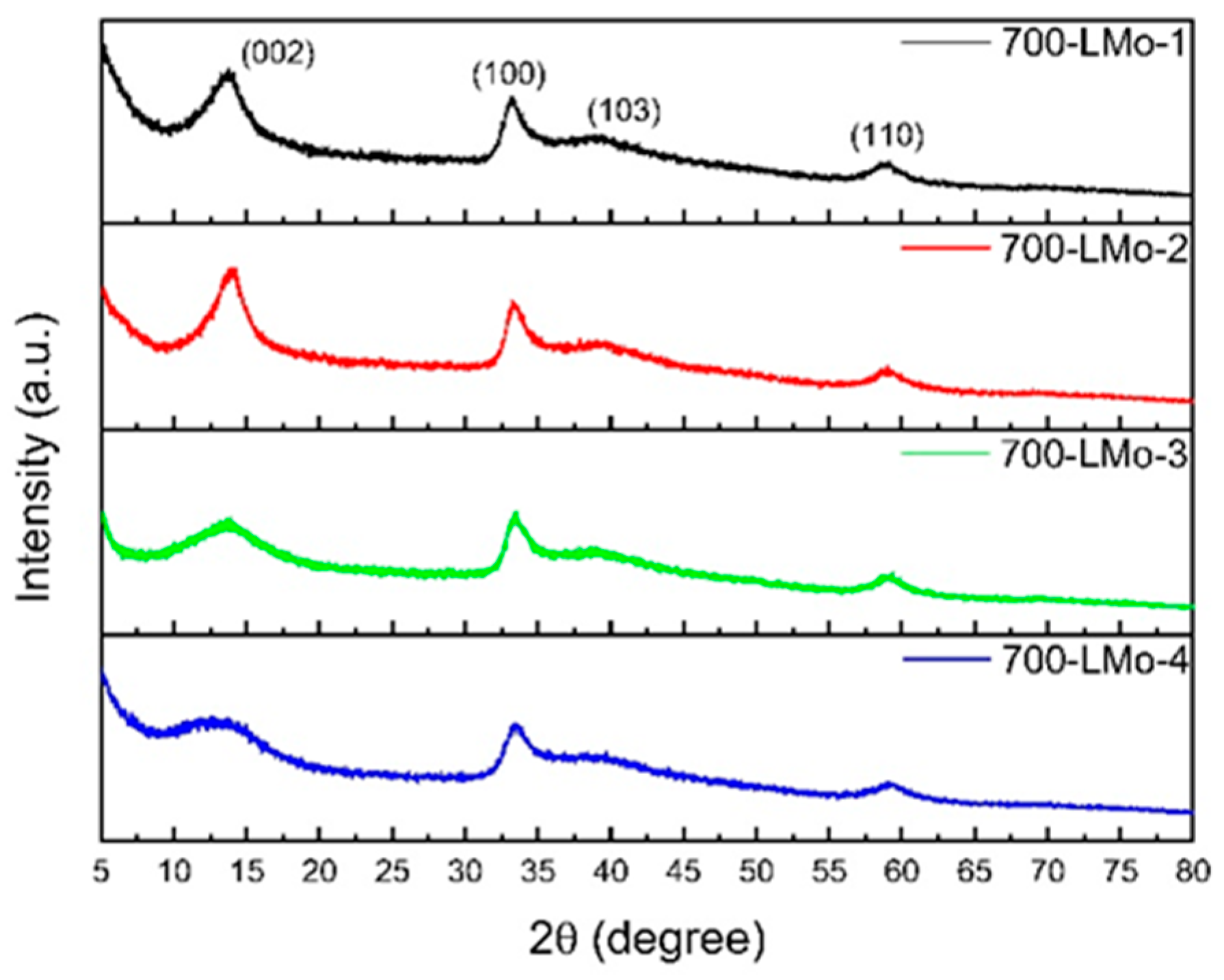

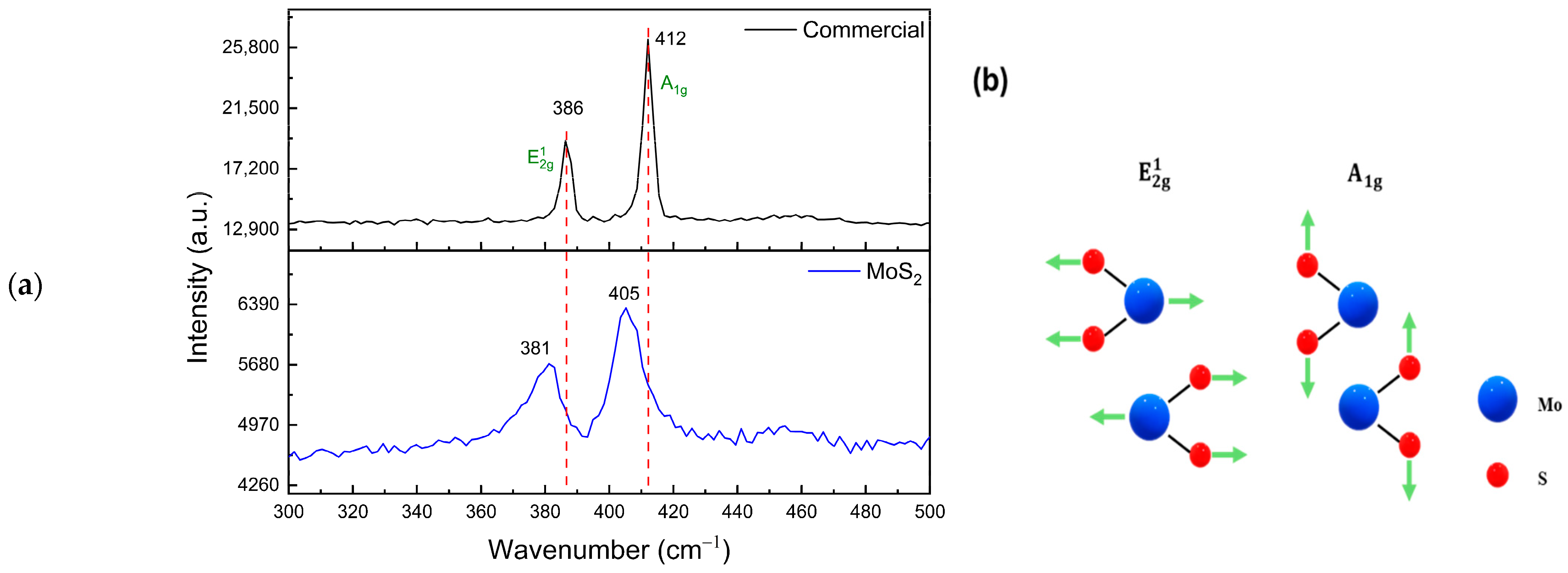
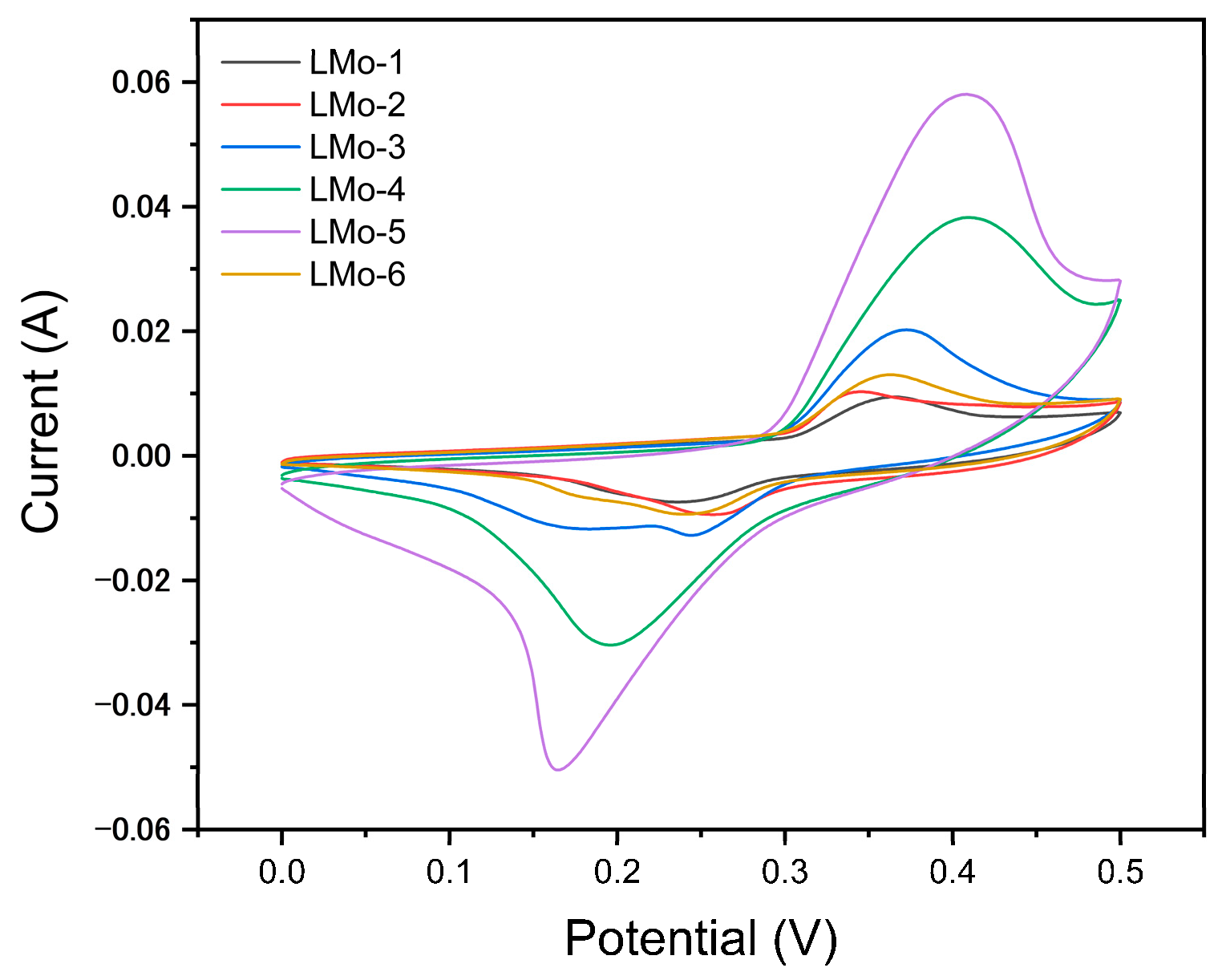
| Sample | LMo-1 | LMo-2 | LMo-3 | LMo-4 | LMo-5 | LMo-6 |
|---|---|---|---|---|---|---|
| Time (hr) | 6 | 12 | 18 | 24 | 24 | 24 |
| pH | 2 | 2 | 2 | 2 | 0.6 | 9.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.-S.; Hu, Y.; Li, S.-Y.; Mazurkiewicz-Pawlicka, M.; Małolepszy, A. Preparation and Electrochemical Properties of Molybdenum Disulfide Nanomaterials. Eng. Proc. 2025, 89, 34. https://doi.org/10.3390/engproc2025089034
Chen P-S, Hu Y, Li S-Y, Mazurkiewicz-Pawlicka M, Małolepszy A. Preparation and Electrochemical Properties of Molybdenum Disulfide Nanomaterials. Engineering Proceedings. 2025; 89(1):34. https://doi.org/10.3390/engproc2025089034
Chicago/Turabian StyleChen, Pin-Syuan, Yi Hu, Si-Ying Li, Marta Mazurkiewicz-Pawlicka, and Artur Małolepszy. 2025. "Preparation and Electrochemical Properties of Molybdenum Disulfide Nanomaterials" Engineering Proceedings 89, no. 1: 34. https://doi.org/10.3390/engproc2025089034
APA StyleChen, P.-S., Hu, Y., Li, S.-Y., Mazurkiewicz-Pawlicka, M., & Małolepszy, A. (2025). Preparation and Electrochemical Properties of Molybdenum Disulfide Nanomaterials. Engineering Proceedings, 89(1), 34. https://doi.org/10.3390/engproc2025089034






