Abstract
This work presents biodiesel production using waste margarine oil and response surface methodology (RSM) for optimisation. The transesterification of waste margarine oil was carried out using sodium hydroxide (NaOH) as a catalyst under atmospheric pressure in a lab-scale batch reactor. Central composite design (CCD) was used to optimise four parameters: methanol-to-oil ratio (3–15 mol/mol), catalyst ratio (0.3–1.5 wt.%), reaction time (30–90 min), and reaction temperature (30–70 °C). Numeral optimisation was performed, and an optimum yield of 99.1% was obtained at an 11.906 methanol-to-oil mol ratio, 1.113 wt.% catalyst ratio, 59.646 min reaction time, 52.459 °C temperature, and a low percentage error yield of 0.942%. Analysis of variance (ANOVA) showed that the methanol-to-oil ratio had the highest influence on the biodiesel yield, followed by the catalyst ratio, and reaction time had the least impact after temperature. The kinetics study revealed that the reaction is controlled by a pseudo-first order, and the activation energy was found to be 62.41 kJ/mol. It was concluded that biodiesel could be produced using waste margarine oil as a cost-effective feedstock optimised by RSM.
1. Introduction
The current industrial revolution and population growth have led to high energy demands, heavily relying on fossil fuels [1,2,3] due to a depletion of these fuels, environmental pollution, and global warming [4,5,6]. This has increased worldwide awareness of environmental conservation and the need for alternative fuels. From the year 2022 to 2023, worldwide energy-related CO2 emissions increased by 1.1% to 37.4 GtCO2. To minimise fossil fuel emissions, the Kyoto Protocol (1997) and the Paris Agreement (2015) have encouraged renewable energy [7]. Therefore, biodiesel has emerged as a viable alternative to petroleum-based gasoline [8]. It is preferred as an alternative fuel because its combustion in diesel engines decreases emissions of hydrocarbons, carbon monoxide, particulate matter, and sulphur dioxide [6]. Additionally, biodiesel exhibits fundamental similarities with traditional petroleum diesel, such as a higher combustion efficiency, a high flash point, and a higher cetane number [2]. Biodiesel is an eco-friendly, biodegradable, and non-toxic fuel [4] synthesised from organic materials such as animal fats, vegetable oil, and waste oils [9]. The range of applications of biodiesel extends well beyond its use in automobiles; it finds usage in power generators, maritime ships, agricultural and mining equipment, boilers, and more [10].
Several methods, such as alcoholysis, micro-emulsion, and pyrolysis, have produced biodiesel. Because of its capacity to produce high-quality biodiesel, alcoholysis, also called esterification or the transesterification reaction, has become the most popular of these processes [2]. Transesterification or esterification, in the presence of a catalyst, produces fatty acid alkyl esters (FAAE), methyl esters when methanol is used, and ethyl esters when ethanol is used [7]. One of the most common approaches to the production of biodiesel is the employment of an acid or basic homogeneous catalyst [11], such as HCl and H2SO4 (acid catalyst) and NaOH and KOH (base catalyst) [12].
The production of economical biodiesel has challenges, such as the high cost of feedstock [11]. Feedstock is a critical component in biofuel manufacturing, comprising 75% of the total production cost [13]. There are four generations of biodiesel feedstock. First-generation feedstocks include edible oils such as palm, sunflower, rapeseed, canola, and soybean. Second-generation feedstocks include non-edible oils such as jatropha, neem, rubber seed, and Karanja. Third-generation feedstocks comprise algae, seaweed, waste frying oils, animal fats, waste oils, and fish oils. The fourth generation encompasses synthetic biology, which remains in a state of development [9]. Using costly edible oils for biodiesel production leads to a conflict between food and fuel, impacting crop yields and land use. As a result, waste feedstocks are being increasingly evaluated for biodiesel production to mitigate elevated edible oil prices [11]. Butter and margarine production factories produce considerable amounts of waste oils. Some of these oils are flushed from production facilities, increasing waste disposal challenges. These margarine oils, a third-generation feedstock, can be two to three times less costly than edible vegetable oils. This drastically lowers processing costs and establishes it as a valuable raw material for producing biodiesel [4].
To reduce costs and maximise the yield of biodiesel production, tools such as Response Surface Methodology (RSM), are commonly employed to optimise the operational parameters of the process [9]. Several researchers used RSM for biodiesel process optimisation. Pugazhendhi et al. [14] investigated the use of RSM for enhanced biodiesel production from waste cooking oil. A maximum yield of 90% was reached in 63 min. By optimising the biodiesel production process, using RSM has reduced operational costs. Ozgur [15] optimised biodiesel production parameters to identify the optimal alcohol, catalyst, and reaction time for transesterification of waste frying oil using RSM, achieving an optimal yield of 93.124%. Yang et al. [16] investigated the optimisation of soybean oil transesterification by the ball-milling method catalyst dosage, reaction time, and rotation speed as process parameters, achieving the optimum yield of 100%. Ao et al. [17] investigated RSM-optimised Jatropha curcas oil transesterification catalysed by active sites engineered biomass-carbon, reaction time, temperature catalyst load, and methanol-to-oil ratio. These process parameters achieved a 97.1% biodiesel yield. Senusi et al. [9] performed an optimisation comparative study of third-generation feedstocks (macroalgae oil, waste palm oil, waste sunflower oil, and waste corn oil), with a maximum yield raging from 89.07% to 99.18%. The molar ratio of methanol-to-oil, catalyst concentration, reaction time, and reaction temperature were the process parameters.
Despite a growing body of literature on biodiesel production, a significant research gap remains in optimising biodiesel derived from waste margarine oil. Most existing studies focus on conventional feedstocks, including vegetable oils, waste vegetable oils, and animal fats, resulting in a notable gap in understanding the challenges and opportunities associated with waste oils. Furthermore, the current kinetic models may not apply to waste margarine oil’s unique characteristics. This research seeks to bridge this gap by investigating the reaction kinetics of waste margarine oil and optimising process parameters such as catalyst concentration, methanol-to-oil molar ratio, reaction time, and temperature, hence contributing to the body of knowledge in biodiesel production and waste valorisation, paving the way for more efficient and cost-effective processes in renewable energy.
2. Materials and Methods
The waste margarine oil used as feedstock was obtained from a local margarine production facility. Potassium hydroxide (85%) was used as a catalyst for transesterification, methanol (99.5%) was used as an acyl acceptor, and phenolphthalein indicators were procured from ACE (Associate Chemical Enterprises), Johannesburg, South Africa. Using the Design Expert software (version 13.0.1.0), central composite design (CCD) in response surface methodology (RSM) was employed for experimental design, as shown in Table 1.

Table 1.
Experimental design.
Before transesterification, the waste margarine oil was heated to 110 °C for 1 h to remove any moisture present. The oil was subsequently cooled to room temperature. The suitability of KOH as a catalyst for waste margarine oil was assessed by calculating the FFA content of the oil, following a method described in our previous study [18]. The FFA content was found to be below the desired value of 2% (1.87 ± 0.059%); therefore, a one-step transesterification route was selected for the transesterification of waste margarine oil. Biodiesel was produced using approximately 100 g of waste margarine oil in every run. Methanol-to-oil molar ratio, catalyst ratio, reaction time, and temperature were varied according to the experimental design at a constant stirring speed of 450 RPM. The experimental runs and yield obtained for every run are shown in Table S1. The reaction vessel consisted of a two-neck, round-bottom flask, equipped with a condenser and a temperature controller. The condenser was installed to ensure that any evaporated methanol remains within the system during the reaction at temperatures approaching or exceeding the boiling point of methanol. The temperature controller regulated the reaction temperature by automatically adjusting to the predetermined set point [19]. The reaction vessel was placed on a heating plate to maintain the reaction’s thermal conditions. The experimental setup is shown in Figure S1.
The designed amount of the catalyst was dissolved in methanol and then transferred to the reaction vessel, which initially contained oil. When the reaction time elapsed, the product mixture was emptied into a separating funnel, separating the biodiesel and the glycerol. The biodiesel was then washed 3 times with distilled water at 60 °C at a ratio of 1:1 (water/biodiesel) to ensure that any traces of methanol and KOH were washed off from the biodiesel. The washed biodiesel was then dried using a heating plate at 105 °C for 1 h until no trace of water was observed. The biodiesel yield was calculated as per Equation (1). A second-order polynomial regression model was employed to establish a relationship between the biodiesel yield and the transesterification process variables, considering first-order, second-order, and interaction effects, as shown in Equation (2):
where Y is the predicted biodiesel yield, Xi, and Xj represent the parameters, βo is the offset term, βi and βj are linear effects, βij is the first-order interaction effect, and βjj is the squared effect.
To facilitate and simplify the kinetics study of the waste margarine oil transesterification, the following consideration were taken into account: The reaction kinetics was assumed to follow the first-order reaction. The rate constants were determined by considering the total transesterification processes, while excluding any intermediary stages. Le Chatelier’s principle indicates that a significant excess of methanol shifts the transesterification equilibrium in favour of the forward reaction, rendering the reverse reactions negligible. Due to excess of methanol, the methanol concentration was treated as a constant [20]. The kinetics models can be represented by Equations (3)–(10).
where TG is triglyceride, M is methanol, G is glycerol, ME is methyl ester, r is reaction rate, X is biodiesel conversion, k is the rate constant, [TGo] is the initial triglyceride concentration, [TG] is the actual triglyceride concentration, t is time, A is the preexponential factor, Ea is activation energy, R is the gas constant (gas constant = 8.314 kJ/kmol. K), and T is temperature in Kelvin.
3. Results and Discussion
Analysis of variance (ANOVA) was used to evaluate the effect of process variables. As shown in Table 2, the Model F-value of 142.98 indicated the significance of the model further supported by a p-value of less than 0.0001 implying that there is only a 0.01% chance that an F-value this large could occur due to noise. Model terms A, B, C, D, AB, AD, BC, BD, CD, A2, B2, C2, and D2 were significant as they had a p-value of less than 0.05 indicating the reliability and fitness of the model. This was evidenced by the not significant lack of fit with a p-value of 0.0808 less than 0.1 which is statistically required. The methanol-to-oil ratio had more impact on the transesterification reaction, followed by the catalyst ratio, temperature, and lastly time. This is indicated by their F-values as shown in Table 2. The high R2 of 0.997 further indicated the fitness of the model. The predicted R2 of 0.7929 was in reasonable agreement with the adjusted R2 of 0.9900 as shown in the fit statistic Table S2. A difference of 0.197 was obtained in this study which agrees with a statistically required difference of less than 0.2 than is required. Adequate precision of 37.731 indicates an adequate signal. Statistically, a ratio greater than 4 is desirable. Adequate precision is the measure of signal-to-noise ratio. This model can be used to navigate the design space [19]. Regression equations in terms of actual factors and coded factors to predicted biodiesel yield in the specified range of process parameters were obtained as shown in Table S3 and Equation (11), respectively. The predicted yield and the actual yield were found to be closely aligned, as evidenced by data near the point line shown in Figure S2. The equation in terms of actual factors can be used to make predictions about the response for given levels of each factor. The levels should be specified in the original units for each factor. The equation should not be used to determine the relative impact of each factor because the coefficients are scaled to accommodate the units of each factor, and the intercept is not at the centre of the design space.
Y = +89.06 + 7.91 × A + 7.16 × B + 3.64 × C + 7.09 × D + 3.08 × AB + 0.4762 × AC + 2.89 × AD −1.09 × BC + 5.43 × BD + 1.34 × CD − 6.07 × A2 − 4.35 × B2 − 2.74 × C2 − 3.91 × D2

Table 2.
ANOVA for quadratic model.
The equation in terms of coded factors can be used to make predictions about the response for given levels of each factor. The coded equation is useful for identifying the relative impact of the factors by comparing the factor coefficients.
Figure 1 and Figure S3 depict the 3D and contour plots of the effects of process parameters on biodiesel yield; (a) shows the interaction effect of the methanol-to-oil ratio and the catalyst ratio at a constant temperature of 50 °C and 60 min; (b) shows the effect of the interaction of the methanol-to-oil ratio and time at a constant temperature of 50 °C and a catalyst ratio of 0.9 wt. %; (c) shows the effect of the interaction of the methanol-to-oil ratio and the temperature at a constant time of 60 min and a catalyst ratio of 0.9 wt. %; (d) shows the effect of the interaction of the catalyst ratio and time at a constant methanol-to-oil ratio of 9 and a temperature of 50 °C; (e) shows the effect of the interaction of the catalyst ratio and the temperature at a constant methanol-to-oil ratio of 9 and time of 60 min; and (f) shows the effect of the interaction of temperature and time at a constant methanol-to-oil ratio of 9 and a catalyst ratio of 0.9 wt.%. The methanol-to-oil ratio was varied from 3 to 15 molar ratio. The results showed an increase in yield as methanol was increased by approximately 90% where a decrease was observed above 12 molar ratios. This increasing behaviour was justified by the fact that transesterification required excess alcohol to shift to the product formation. A decrease is justified by a high methanol-to-oil ratio and can lead to glycerolysis [5]. The catalyst was varied from 0.3 to 1.5 wt. %. An increase in yield using a catalyst ratio of up to 1.1 wt. % was observed. The catalyst reached the maximum amount as it is required to facilitate the reaction. The decrease is justified by the excess catalyst leading to saponification, affecting the separation [4,21]. The temperature was varied from 30 to 70 °C. As the temperature was increased, the biodiesel yield increased, attaining the highest yield at temperatures between 50 °C to 60 °C. At above 60 °C, a decrease in yield was observed. The increase in yield behaviour is explained by the temperature increasing the reaction rate as the activation energy increases [19]. The decrease is caused by the evaporation of methanol affecting methanol interaction [17]. Reaction time was varied from 30 to 90 min. The biodiesel yield increased with increased time, reaching a point where there was no more increase observed, and a slight decrease was observed. The increase is justified as a contact time is required for a mass transfer diffusion [5] of methanol into the waste margarine oil to reach equilibrium, and once equilibrium was reached at approximately 70 min, a slight decrease was observed suggesting that a possible reversible reaction occurred [5,17].
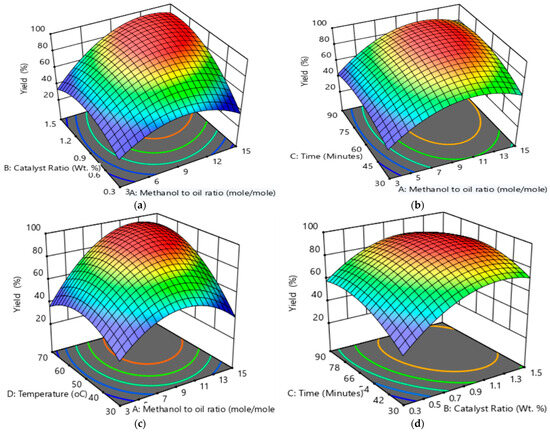
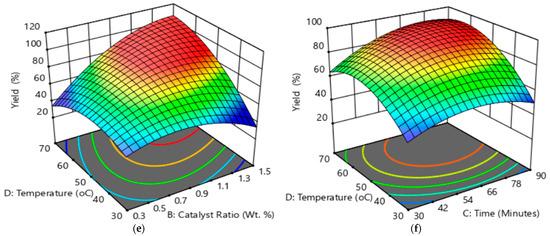
Figure 1.
3D plots of effect of process parameters on biodiesel yield; (a) effect of interaction of methanol-to-oil ratio and catalyst ratio at constant temperature of 50 °C and 60 min; (b) effect of interaction of methanol-to-oil ratio and time at constant temperature of 50 °C and catalyst ratio of 0.9 wt. %; (c) effect of interaction of methanol-to-oil ratio and temperature at constant time of 60 min and catalyst ratio of 0.9 wt. %; (d) effect of interaction of catalyst ratio and time at constant methanol-to-oil ratio of 9 and temperature of 50 °C; (e) effect of interaction of catalyst ratio and temperature at constant methanol-to-oil ratio of 9 and time of 60 min; and (f) effect of interaction of temperature and time at constant methanol-to-oil ratio 9 of and catalyst ratio of 0.9 wt.%. From blue to red, low to high biodiesel yield.
Numerical optimisation was performed in RSM as shown in Table S4. The optimum conditions were selected to be 11.906 methanol-to-oil molar ratio, 1.113 wt. % catalyst ratio, 59.646 min reaction time, and 52.459 °C reaction temperature obtaining the yield of 99.1%, with an error of 0.942%. This solution was selected based on its high yield and low standards of errors. This experiment validated obtaining an experimental yield of 97.67 ± 0.882% which is an acceptable range with the numerical optimum yield.
Figure 2 shows the kinetics and Arrhenius plots used to determine the kinetics. Figure 2a was used to obtain a rate constant at different temperatures, which was later used in logarithm and temperature, the significant linearity between lnk nd 1000/T over the 303–333 K (30–60 °C). The Ea was determined by the rate at which the k constant changes with temperature, as shown in Figure 2b and using Equation (10). The approximate activation energy of 62.41 kJ∙mol−1 was obtained. The activation energy obtained is close to what was reported by Mwenge et al. [5] and in the range of 24.7 to 84.1 kJ∙mol−1 obtained by other researchers [17].
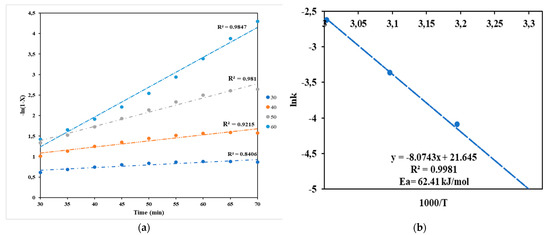
Figure 2.
Kinetics plots: (a) plot of -lnk (1-X) vs. reaction time at different temperatures; (b) Arrhenius plot lnk vs. 1000/T for the transesterification reaction of waste margarine oil.
The biodiesel sample produced under optimum conditions was analysed to assess its compliance with ASTM D6751 standards [18]. The results shown in Table 3 indicate that all key fuel properties meet the required specifications. The density of 0.8687 g/cm3 falls within the acceptable range of 0.86–0.90 g/cm3, ensuring proper fuel atomisation and combustion. The flash point (148 °C) exceeds the minimum threshold (>130 °C), indicating safe handling and storage. The viscosity (4.6503 mm2/s) is within the recommended range (1.9–6.0 mm2/s), ensuring smooth fuel flow. Additionally, the water content (0.0295%), sulphur content (3.32 mg/kg), and cetane number (56) comply with ASTM standards, confirming the biodiesel’s quality and efficiency.

Table 3.
Waste margarine biodiesel fuel properties.
4. Conclusions
This work investigated the optimisation of biodiesel production from waste margarine oil as a promising path for advancing renewable energy technologies. By addressing the kinetic aspects of the transesterification process, this work aimed to fill knowledge gaps in the literature and provide insights for the practical implementation of biodiesel production from waste oils. A numerical optimum yield of 99.1% was obtained at an 11.906 mol methanol ratio, 1.113 wt. % catalyst ratio, 59.646 min and 52.459 °C, and a low standard error of 0.942%, which was experimentally validated, obtaining a mean value of 97.64%. ANOVA showed that the methanol-to-oil ratio had the highest influence on the biodiesel yield, followed by the catalyst ratio, and reaction time had the least impact after temperature. The kinetics study was performed to obtain the activation energy of 62.41 kJ/mol. The produced biodiesel conformed to ASTM specifications. It was concluded that biodiesel could be produced using waste margarine oil as a cost-effective feedstock optimised by RSM, promoting the circular economy and enhancing the viability of biodiesel as a renewable energy source.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/engproc2025087012/s1, Table S1: Experimental runs with yield; Table S2: Fit statistics; Table S3: Final equation in terms of actual factors; Table S4: Optimisation solutions; Figure S1: Experimental setup; Figure S2: Plot of actual vs. predicted yield; Figure S3: Contour plot of effect of process parameters on biodiesel yield.
Author Contributions
Conceptualisation, P.M.; methodology, P.M. and S.M.; software, P.M.; validation, P.M., S.M. and H.R.; formal analysis, P.M., S.M. and H.R.; investigation, P.M., S.M. and H.R.; resources, P.M., SM. and H.R.; data curation, P.M.; writing—original draft preparation, P.M. and S.M.; writing—review and editing, P.M., S.M. and H.R.; visualisation, P.M., S.M. and H.R.; project administration, H.R.; funding acquisition, H.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting the findings of this study are available within the article and its Supplementary Materials.
Acknowledgments
The authors thank the Department of Chemical and Metallurgical Engineering of the Vaal University of Technology for providing research facilities.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mwenge, P.; Rutto, H.; Seodigeng, T. Application of Machine Learning for Methanolysis of Waste Cooking Oil Using Kaolinite Geopolymer Heterogeneous Catalyst. In Proceedings of the 3rd International Electronic Conference on Processes, Online, 29–31 May 2024; p. 23. [Google Scholar]
- Esmi, F.; Dalai, A.K.; Hu, Y. Comparison of Various Machine Learning Techniques for Modeling the Heterogeneous Acid-Catalyzed Alcoholysis Process of Biodiesel Production from Green Seed Canola Oil. Energy Rep. 2024, 12, 321–328. [Google Scholar] [CrossRef]
- Cerón Ferrusca, M.; Romero, R.; Martínez, S.L.; Ramírez-Serrano, A.; Natividad, R. Biodiesel Production from Waste Cooking Oil: A Perspective on Catalytic Processes. Processes 2023, 11, 1952. [Google Scholar] [CrossRef]
- Mwenge, P.; Rutto, H.; Seodigeng, T. Modelling and Optimisation of Biodiesel Production from Margarine Waste Oil Using a Three-Dimensional Machine Learning Approach. In Proceedings of the 3rd International Electronic Conference on Processes, Online, 29–31 May 2024; p. 27. [Google Scholar]
- Mwenge, P.; Rutto, H.; Enweremadu, C. Biodiesel Production Using Chlor-Alkali Brine Sludge Waste as a Heterogeneous Catalyst: Optimisation Using Response Surface Methodology. Int. J. Sustain. Energy 2022, 41, 832–845. [Google Scholar] [CrossRef]
- Ali, M.; Shahid, M.; Saeed, W.; Imran, S.; Kalam, M.A. Design, Fabrication, and Operation of a 10 L Biodiesel Production Unit Powered by Conventional and Solar Energy Systems. Sustainability 2023, 15, 9734. [Google Scholar] [CrossRef]
- Prajapati, N.; Singh Kachhwaha, S.; Kodgire, P.; Kumar Vij, R. A Novel High-Speed Homogenizer Assisted Process Intensification Technique for Biodiesel Production Using Soya Acid Oil: Process Optimization, Kinetic and Thermodynamic Modelling. Energy Convers. Manag. 2025, 324, 119302. [Google Scholar] [CrossRef]
- Mwenge, P.; Rutto, H. Machine Learning-Based Predictive Modelling of Biodiesel Production from Animal Fats Catalysed by a Blast Furnace Slag Geopolymer. Results Eng. 2025, 25, 104126. [Google Scholar] [CrossRef]
- Senusi, W.; Ahmad, M.I.; Abdul Khalil, H.P.S.; Shakir, M.A.; Binhweel, F.; Shalfoh, E.; Alsaadi, S. Comparative Assessment for Biodiesel Production from Low-Cost Feedstocks of Third Oil Generation. Renew. Energy 2024, 236, 121369. [Google Scholar] [CrossRef]
- Ghosh, N.; Halder, G. Current Progress and Perspective of Heterogeneous Nanocatalytic Transesterification towards Biodiesel Production from Edible and Inedible Feedstock: A Review. Energy Convers. Manag. 2022, 270, 116292. [Google Scholar] [CrossRef]
- Elgharbawy, A.S.; Osman, A.I.; El Demerdash, A.G.M.; Sadik, W.A.; Kasaby, M.A.; Ali, S.E. Enhancing Biodiesel Production Efficiency with Industrial Waste-Derived Catalysts: Techno-Economic Analysis of Microwave and Ultrasonic Transesterification Methods. Energy Convers. Manag. 2024, 321, 118945. [Google Scholar] [CrossRef]
- Alsultan, A.G.; Asikin-Mijan, N.; Ibrahim, Z.; Yunus, R.; Razali, S.Z.; Mansir, N.; Islam, A.; Seenivasagam, S.; Taufiq-Yap, Y.H. A Short Review on Catalyst, Feedstock, Modernised Process, Current State and Challenges on Biodiesel Production. Catalysts 2021, 11, 1261. [Google Scholar] [CrossRef]
- Suhara, A.; Karyadi; Herawan, S.G.; Tirta, A.; Idris, M.; Roslan, M.F.; Putra, N.R.; Hananto, A.L.; Veza, I. Biodiesel Sustainability: Review of Progress and Challenges of Biodiesel as Sustainable Biofuel. Clean Technol. 2024, 6, 886–906. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Alagumalai, A.; Mathimani, T.; Atabani, A.E. Optimization, Kinetic and Thermodynamic Studies on Sustainable Biodiesel Production from Waste Cooking Oil: An Indian Perspective. Fuel 2020, 273, 117725. [Google Scholar] [CrossRef]
- Özgür, C. Optimization of Biodiesel Yield and Diesel Engine Performance from Waste Cooking Oil by Response Surface Method (RSM). Pet. Sci. Technol. 2021, 39, 683–703. [Google Scholar] [CrossRef]
- Yang, N.; Sheng, X.; Ti, L.; Jia, H.; Ping, Q.; Li, N. Ball-Milling Transesterification Process on Biodiesel Production: RSM Optimization, Life Cycle Assessment and Market Dynamics Analysis. Energy 2023, 283, 129201. [Google Scholar] [CrossRef]
- Ao, S.; Gouda, S.P.; Selvaraj, M.; Boddula, R.; Al-Qahtani, N.; Mohan, S.; Rokhum, S.L. Active Sites Engineered Biomass-Carbon as a Catalyst for Biodiesel Production: Process Optimisation Using RSM and Life Cycle Assessment. Energy Convers. Manag. 2024, 300, 117956. [Google Scholar] [CrossRef]
- Mwenge, P.; Dlamini, N.; Rutto, H.; Seodigeng, T. Biodiesel Production from Waste Margarine Using Homogeneous Catalyst Potassium Hydroxide. In Proceedings of the 14th PARIS International Conference on Agriculture, Biological & Environmental Sciences (PABE-19), Paris, France, 5 September 2019; pp. 33–37. [Google Scholar]
- Mwenge, P.; Luboya, A.; Muthubi, S.; Rutto, H.; Seodigeng, T. Biodiesel Production from Animal Fats Using Blast Furnace Geopolymer Heterogeneous Catalyst: Optimisation and Kinetic Study. Arab. J. Sci. Eng. 2025. [Google Scholar] [CrossRef]
- Mwenge, P.; Djemima, B.; Zwane, S.; Muthubi, S.; Rutto, H.; Seodigeng, T. Kinetics and Simulation of Biodiesel Production Using a Geopolymer Heterogenous Catalyst. J. Environ. Sci. Health Part A 2024, 59, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Mwenge, P.; Rutto, H.; Enweremadu, C. Production of Biodiesel Using Calcined Brine Sludge Waste from Chor-Alkali Industry as a Heterogeneous Catalyst. Environ. Clim. Technol. 2021, 25, 621–630. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).