Analysis of Natural Vaporization in LPG Tanks †
Abstract
1. Introduction
2. Methods
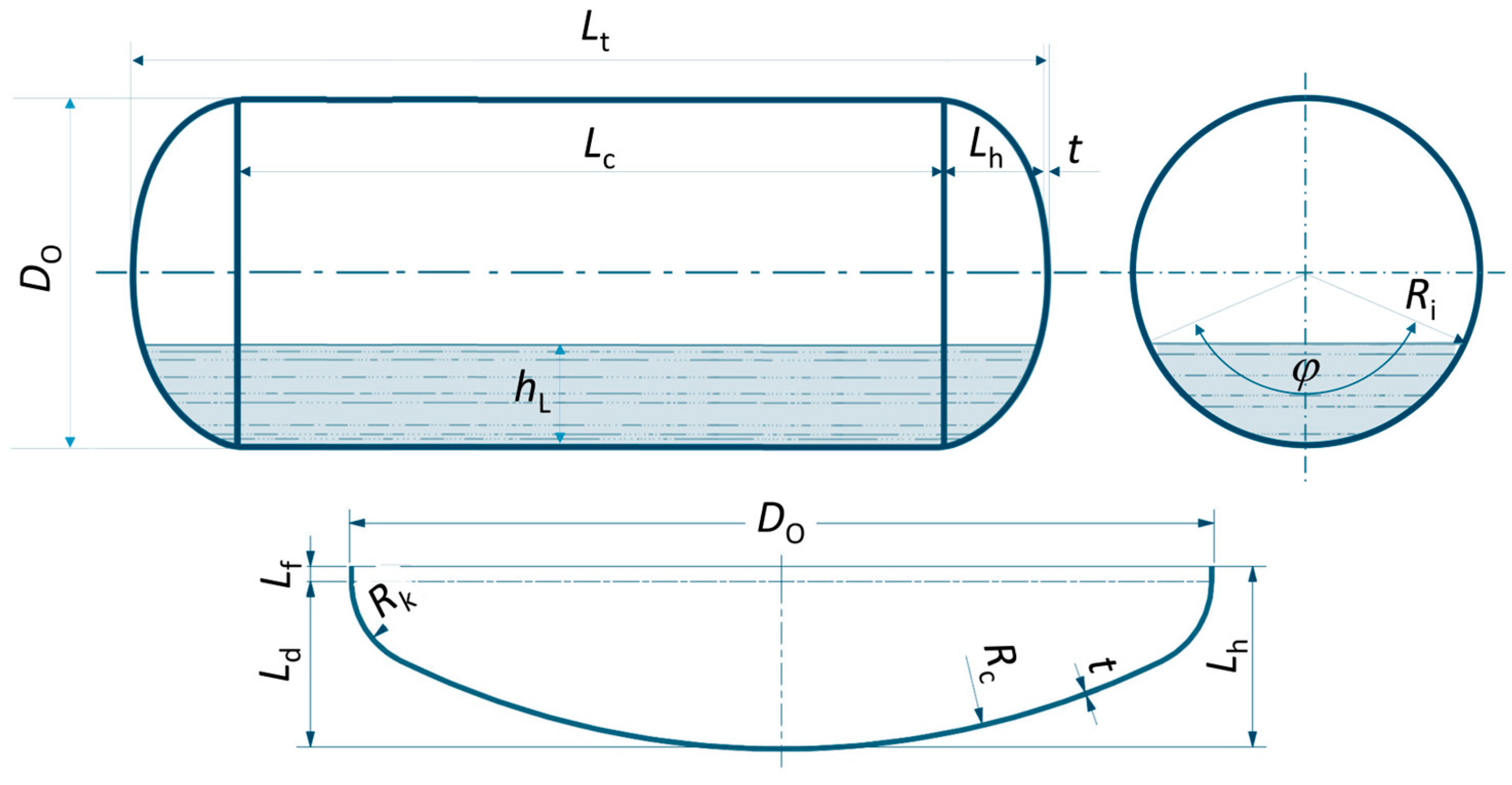
2.1. Geometrical Properties
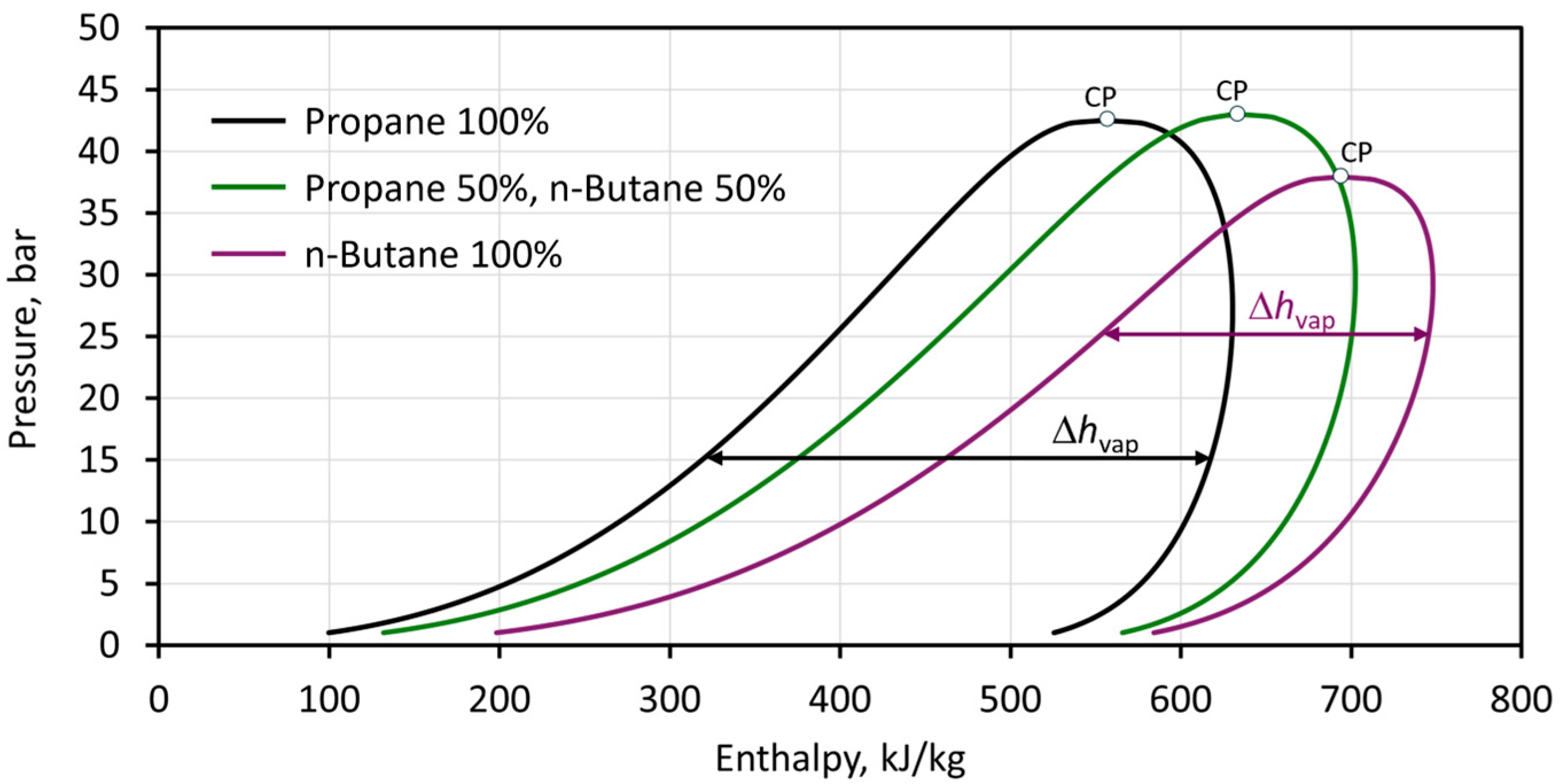
2.2. Heat Transfer Properties
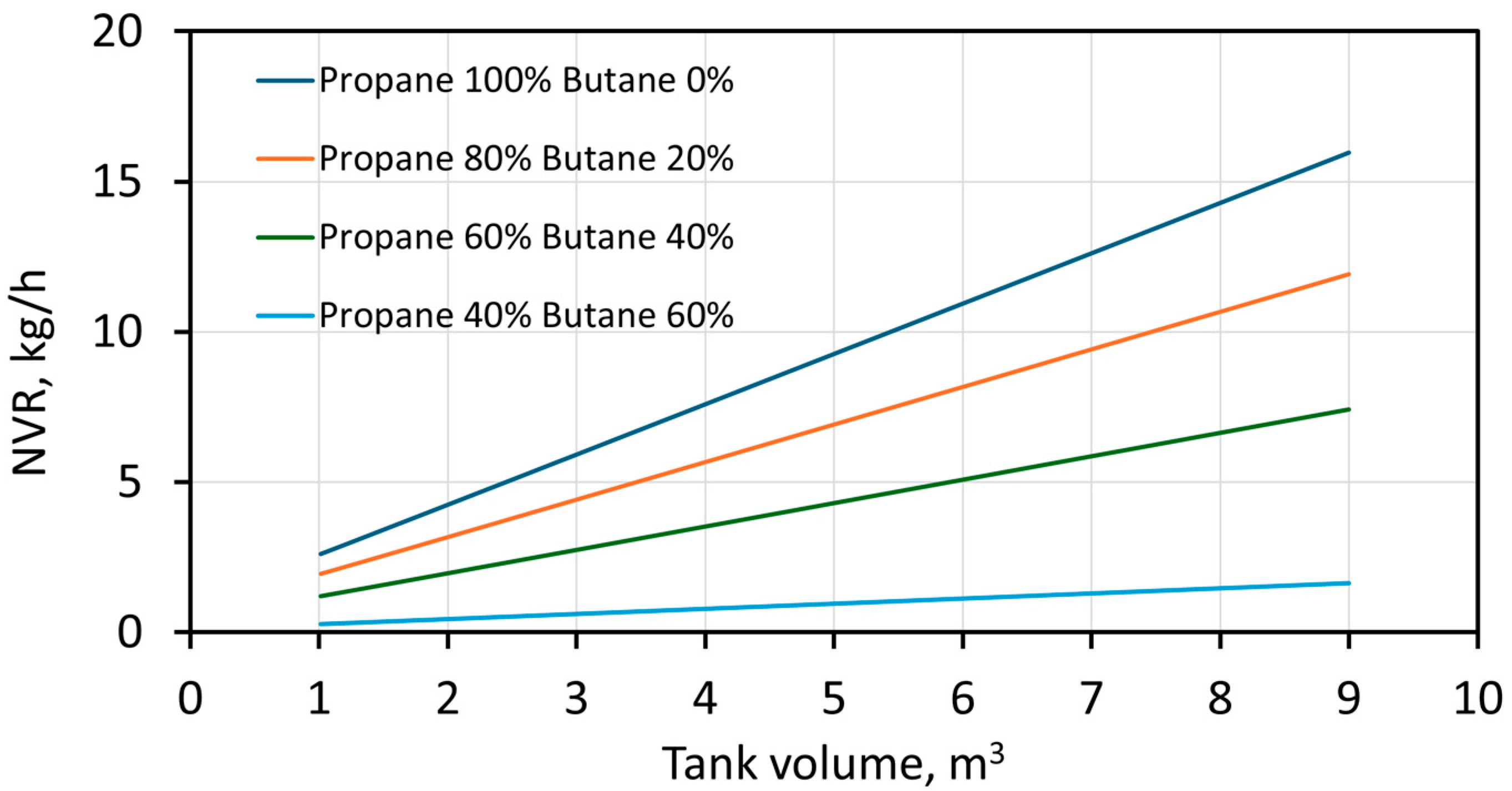
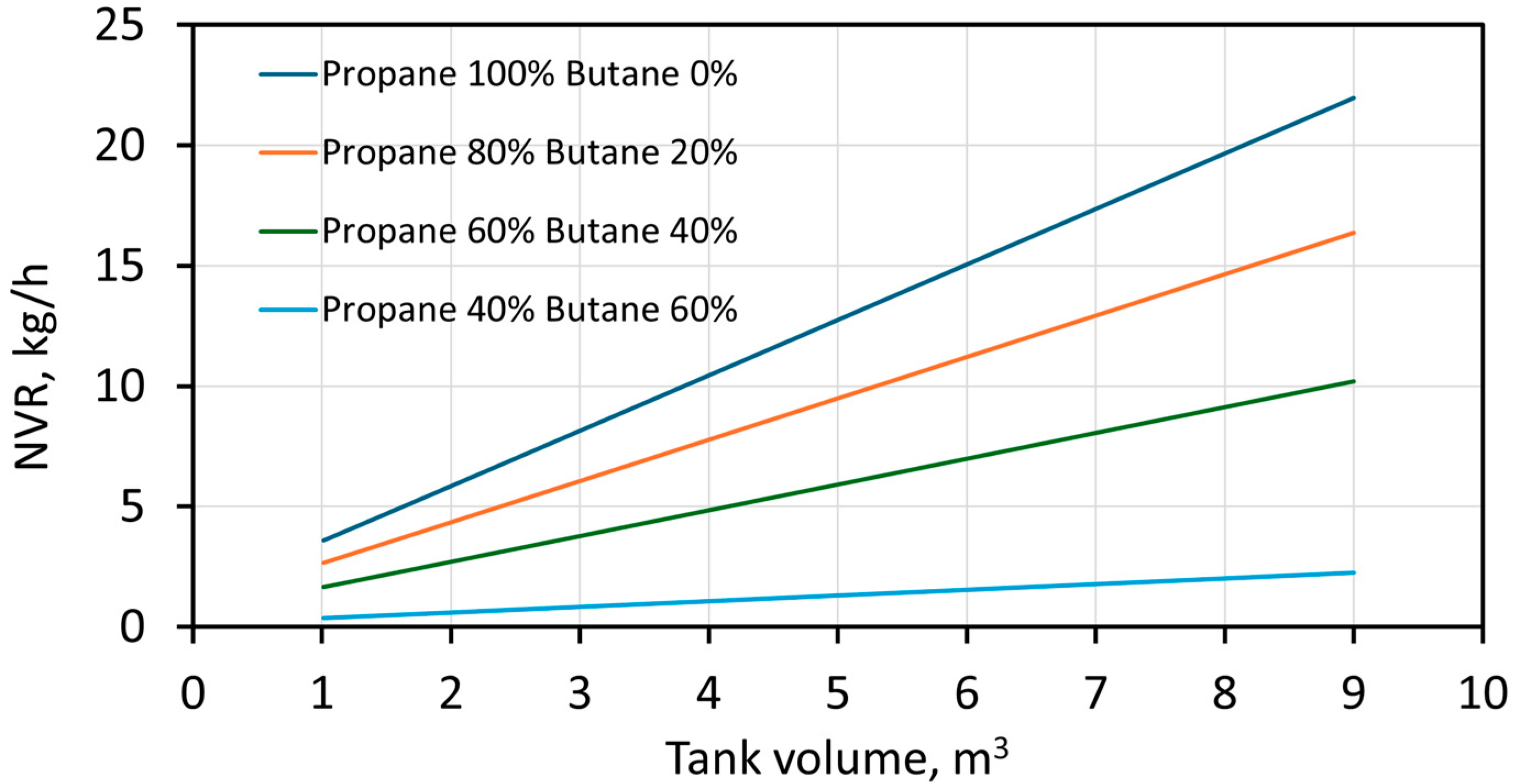
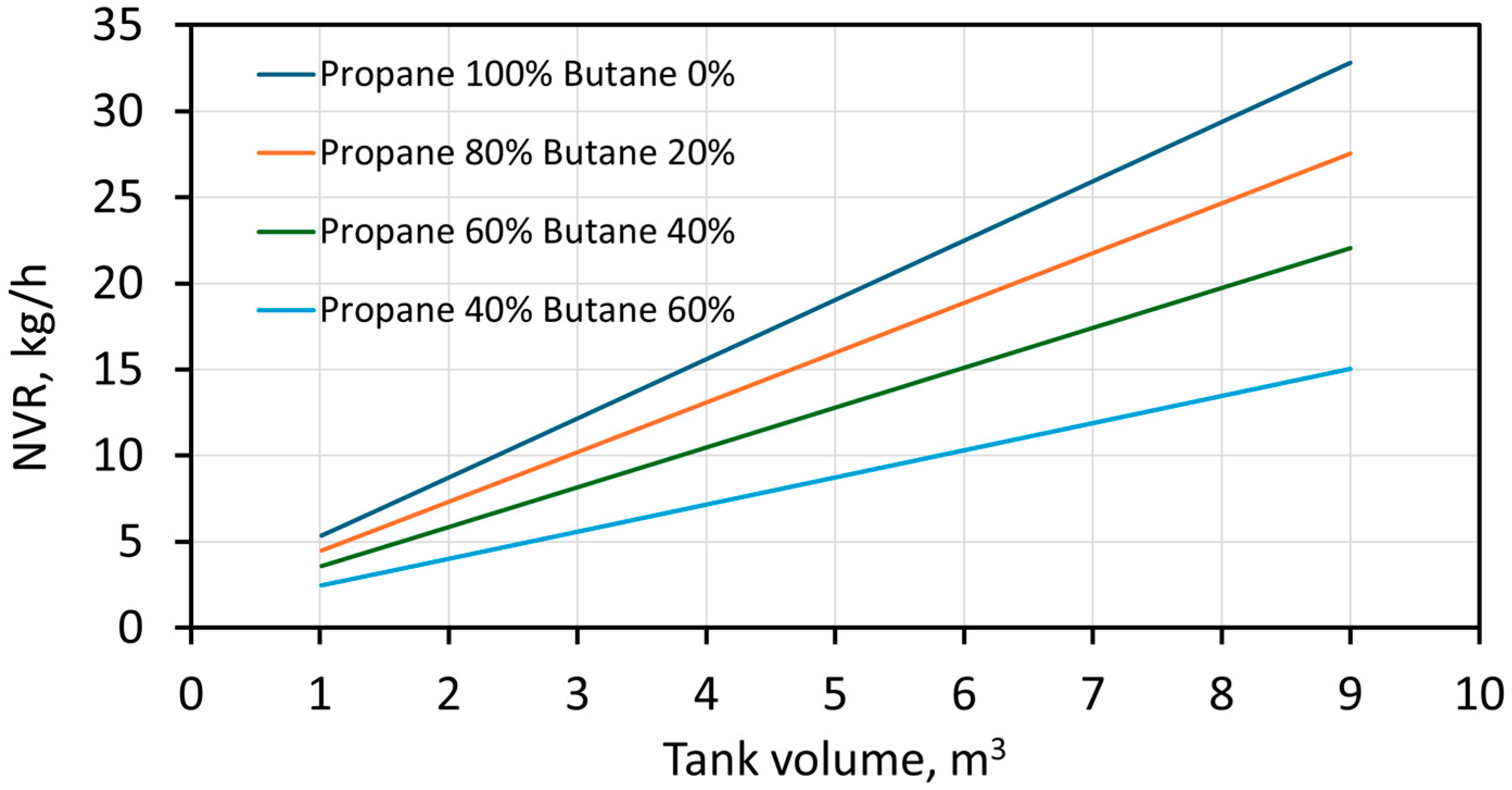
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Green Gas: LPG Basic Principles and Practices. Available online: https://greengasjo.com/files/Gas%20Networks%20-Technical%20Overview.pdf (accessed on 10 January 2025).
- Morozyuk, L.; Kosoy, B.; Sokolovska-Yefymenko, V.; Ierin, V. Analysis of Mixing Processes of LPG Gases in Tanks When Transporting by Sea. Dynamics 2022, 2, 219–233. [Google Scholar] [CrossRef]
- Emrys Scarponi, G.; Landucci, G.; Heymes, F.; Cozzani, V. Experimental and numerical study of the behavior of LPG tanks exposed to wildland fires. Process Saf. Environ. Prot. 2018, 114, 251–270. [Google Scholar] [CrossRef]
- Ma, Q.; Zhong, M.; Guo, Y.; You, J.; He, Y.; Chen, J.; Zhang, Z. Study on the characteristic of boiling expansion process of superheated LPG and its vapor cloud explosion. J. Loss Prev. Process Ind. 2022, 78, 104831. [Google Scholar] [CrossRef]
- Lapesa: LPG Tanks with Atmospheric Vaporizer. Available online: https://www.lapesa.es/en/lpg-tanks-atmospheric-vaporizer (accessed on 10 January 2025).
- Lapesa: Equipment for LPG—Forced Vaporization. Available online: https://www.lapesa.es/sites/default/files/modular_equipment_vaporization_and_heating.pdf (accessed on 10 January 2025).
- Vasiliev, L.L.; Khrolenok, V.V.; Zhuravlyov, A.S. Intensification of heat transfer at propane pool boiling on single horizontal tubes. Rev. Gén. Therm. 1998, 37, 962–967. [Google Scholar] [CrossRef]
- Chiang, C.H.; Raju, M.S.; Sirignano, W.A. Numerical analysis of convecting, vaporizing fuel droplet with variable properties. Int. J. Heat Mass Transf. 1992, 35, 1307–1324. [Google Scholar] [CrossRef]
- Oh, S.J.; Kwon, J.; Jeon, K.S.; Yoon, J.H. A Numerical Analysis Study on the Characteristics of Evaporation in Liquid Hydrogen Tank with Vacuum Layer According to Changes in Heat Flux and Vacuum Pressure. Int. J. Hydrogen Energy 2024, 50, 542–557. [Google Scholar] [CrossRef]
- Ejeh, C.J.; Akhabue, G.P.; Agyeibi, I. Effect of Transient Temperature on 304 Stainless Steel LPG Tank Structure Using Numerical Simulation Approach. SN Appl. Sci. 2019, 1, 1690. [Google Scholar] [CrossRef]
- Lomazzi, L.; Passoni, S.; Mereu, R.; Cadini, F.; Giglio, M. In-Depth Analysis of the Burst of a Liquefied Petroleum Gas Tank in Gravedona, Italy. Energies 2024, 17, 3741. [Google Scholar] [CrossRef]
- Pavković, B.; Čarija, Z.; Samardžić, I. Analysis of Propane Thermal State in Underground Tanks Taking into Consideration Design Issues. Strojarstvo 2008, 50, 387–394. [Google Scholar]
- RedRiver: Exploring the Four Primary Head Types for Pressure Vessels. Available online: https://www.redriver.team/exploring-the-four-primary-head-types-for-pressure-vessels/ (accessed on 10 January 2025).
- Wolfram MathWorld: Torispherical Dome. Available online: https://mathworld.wolfram.com/TorisphericalDome.html (accessed on 10 January 2025).
- BS EN 12542:2020; LPG Equipment and Accessories—Static Welded Steel Cylindrical Pressure Vessels, Serially Produced for the Storage of Liquefied Petroleum Gas (LPG) Having a Volume not Greater Than 13 m3—Design and Manufacture. British Standards Institution (BSI): London, UK, 2020.
- BS EN 10028-2:2017; Flat Products Made of Steels for Pressure Purposes—Non-Alloy and Alloy Steels with Specified Elevated Temperature Properties. British Standards Institution (BSI): London, UK, 2017.
- EN 10273 Grade P265GH normalized or normalized formed (+N). Available online: https://www.shipplatesteelpro.com/EN-10273-Grade-P265GH.html (accessed on 21 April 2025).
- Cengel, Y.; Ghajar, A. Heat and Mass Transfer: Fundamentals and Applications, 6th ed.; McGraw Hill: New York, NY, USA, 2020. [Google Scholar]
- Bejan, A.; Kraus, A.D. Heat Transfer Handbook; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003. [Google Scholar]
- Stephan, K.; Abdelsalam, M. Heat transfer correlations for natural convection boiling. Int. J. Heat Mass Transf. 1980, 23, 73–87. [Google Scholar] [CrossRef]
- Huber, M.L.; Lemmon, E.W.; Bell, I.H.; McLinden, M.O. The NIST REFPROP Database for Highly Accurate Properties of Industrially Important Fluids. Ind. Eng. Chem. Res. 2022, 61, 7858–7873. [Google Scholar] [CrossRef] [PubMed]
- Bell, I.H.; Wronski, J.; Quoilin, S.; Lemort, V. Pure and Pseudo-pure Fluid Thermophysical Property Evaluation and the Open-Source Thermophysical Property Library CoolProp. Ind. Eng. Chem. Res. 2014, 53, 2498–2508. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pokorny, F.; Blecich, P.; Bonefačić, I. Analysis of Natural Vaporization in LPG Tanks. Eng. Proc. 2025, 87, 98. https://doi.org/10.3390/engproc2025087098
Pokorny F, Blecich P, Bonefačić I. Analysis of Natural Vaporization in LPG Tanks. Engineering Proceedings. 2025; 87(1):98. https://doi.org/10.3390/engproc2025087098
Chicago/Turabian StylePokorny, Filip, Paolo Blecich, and Igor Bonefačić. 2025. "Analysis of Natural Vaporization in LPG Tanks" Engineering Proceedings 87, no. 1: 98. https://doi.org/10.3390/engproc2025087098
APA StylePokorny, F., Blecich, P., & Bonefačić, I. (2025). Analysis of Natural Vaporization in LPG Tanks. Engineering Proceedings, 87(1), 98. https://doi.org/10.3390/engproc2025087098





