Nanoscopic Characterization of Reduced Graphene Oxide for Anticorrosion Coating of AA2024 †
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. AFM Measurements
2.4. Micro-Raman Setup
2.5. Electrochemical Experiments Setup
3. Results and Discussion
3.1. Topography Characteristics
3.2. Micro-Raman Measurements
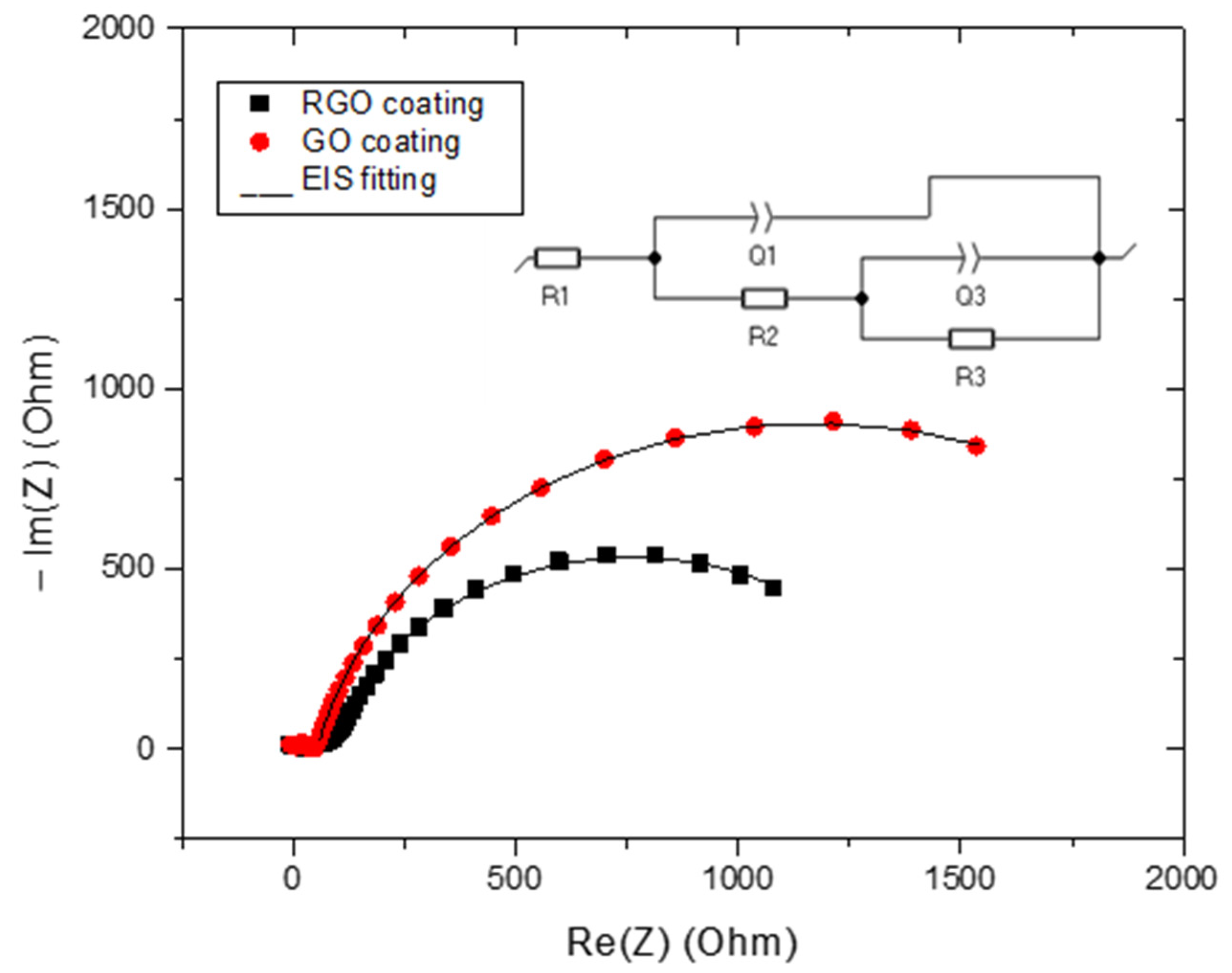
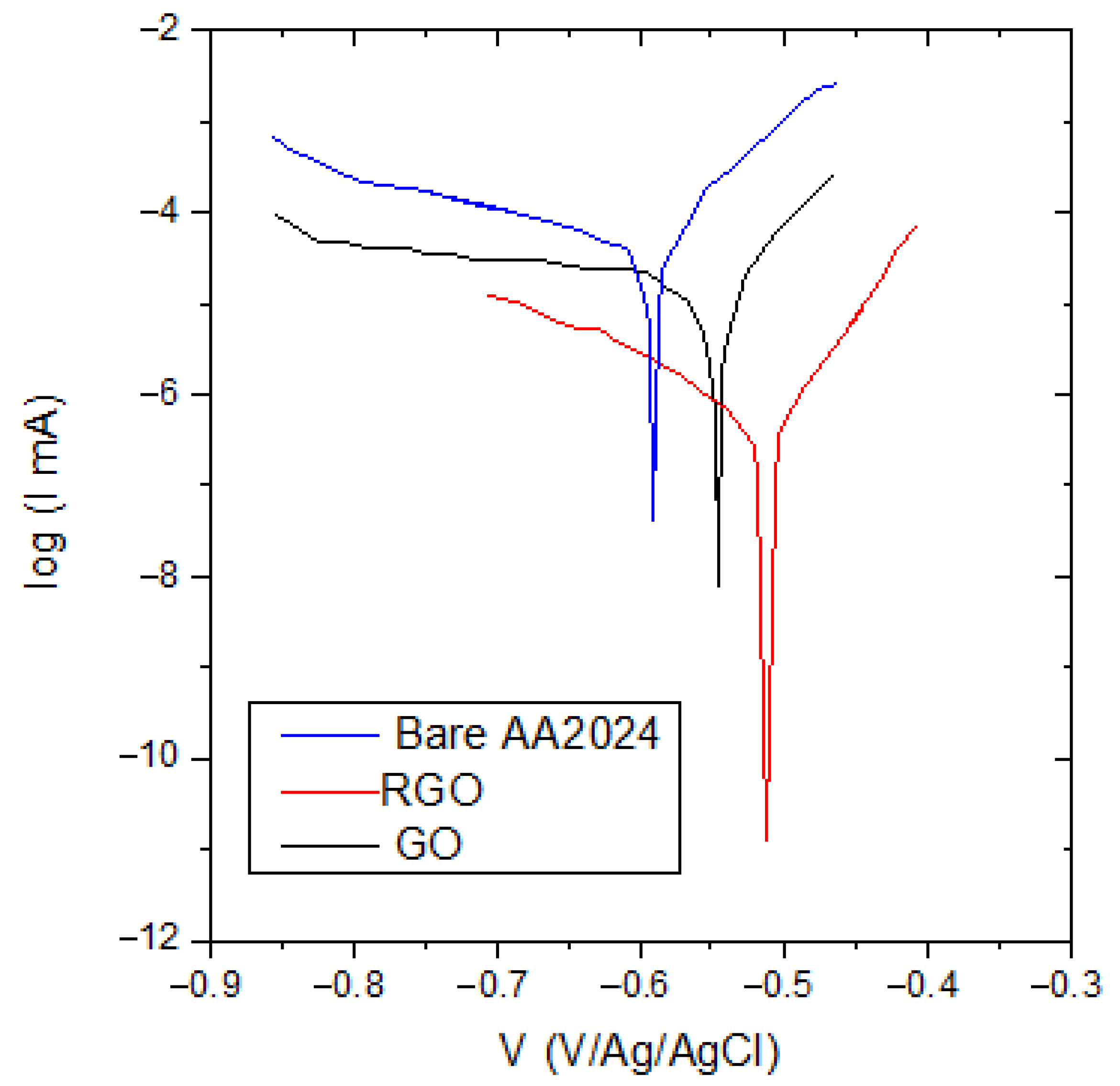
3.3. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Olgiati, M.; Denissen, P.J.; Garcia, S.J. When All Intermetallics Dealloy in AA2024-T3: Quantifying Early Stage Intermetallic Corrosion Kinetics under Immersion. Corros. Sci. 2021, 192, 109836. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, D.B.; Cui, J.P.; Li, T.R.; Emori, W.; Zhang, S.D.; Wang, J.Q. Corrosion Susceptibility of the Nanophases of the Al-Based Amorphous-Nanocrystalline Coatings. Corros. Sci. 2023, 223, 111474. [Google Scholar] [CrossRef]
- Shi, Y.; Collins, L.; Balke, N.; Liaw, P.K.; Yang, B. In-Situ Electrochemical-AFM Study of Localized Corrosion of AlxCoCrFeNi High-Entropy Alloys in Chloride Solution. Appl. Surf. Sci. 2018, 439, 533–544. [Google Scholar] [CrossRef]
- Kreta, A.; Pavlica, E.; Božič, M.; Bratina, G. Nanoscopic Roughness Characterization of Chitosan with Buried Graphene Oxide for Fuel Cell Application. Eng. Proc. 2023, 31, 26. [Google Scholar]
- Biswas, S.; Nath, A.; Pal, A. Application of Graphene, Graphene Oxide and Reduced Graphene Oxide Based Composites for Removal of Chlorophenols from Aqueous Media. In Graphene and Its Derivatives (Volume 2). Materials Horizons: From Nature to Nanomaterials; Mohanty, K., Saran, S., Kumara Swamy, B.E., Sharma, S.C., Eds.; Springer: Singapore, 2023. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and Graphene Oxide: Synthesis, Properties, and Applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Smith, A.T.; LaChance, A.M.; Zeng, S.; Liu, B.; Sun, L. Synthesis, Properties, and Applications of Graphene Oxide/Reduced Graphene Oxide and Their Nanocomposites. Nano Mater. Sci. 2019, 1, 31–47. [Google Scholar] [CrossRef]
- Kim, H.W.; Yoon, H.W.; Yoon, S.M.; Yoo, B.M.; Ahn, B.K.; Cho, Y.H.; Shin, H.J.; Yang, H.; Paik, U.; Kwon, S.; et al. Selective Gas Transport through Few-Layered Graphene and Graphene Oxide Membranes. Science (1979) 2013, 342, 91–95. [Google Scholar] [CrossRef]
- Yang, S.; Lohe, M.R.; Müllen, K.; Feng, X. New-Generation Graphene from Electrochemical Approaches: Production and Applications. Adv. Mater. 2016, 28, 6213–6221. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the Elastic Properties and Intrinsic Strength of Monolayer Graphene. Science (1979) 2008, 321, 385–388. [Google Scholar] [CrossRef]
- Cheng, C.; Li, S.; Thomas, A.; Kotov, N.A.; Haag, R. Functional Graphene Nanomaterials Based Architectures: Biointeractions, Fabrications, and Emerging Biological Applications. Chem. Rev. 2017, 117, 1826–1914. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Liu, G.; Huang, K.; Chu, Z.; Jin, W.; Xu, N. Subnanometer Two-Dimensional Graphene Oxide Channels for Ultrafast Gas Sieving. ACS Nano 2016, 10, 3398–3409. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, J.; Wang, W.; Chen, H.; Liu, Z.; Yu, Q.; Zeng, H.; Sun, L. Aqueous Phase Preparation of Graphene with Low Defect Density and Adjustable Layers. Chem. Commun. 2013, 49, 10835–10837. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Abbasi, S.A.; Li, X.; Ho, H.P.; Yuan, W. Dynamic Tunable Liquid-Core Photonic Crystal Fiber Sensor Based on Graphene Plasmon. Plasmonics 2024, 20, 1083–1091. [Google Scholar] [CrossRef]
- Huang, K.; Liu, G.; Lou, Y.; Dong, Z.; Shen, J.; Jin, W. A Graphene Oxide Membrane with Highly Selective Molecular Separation of Aqueous Organic Solution. Angew. Chem. 2014, 126, 7049–7052. [Google Scholar] [CrossRef]
- Wang, S.; Liu, N.; Su, J.; Li, L.; Long, F.; Zou, Z.; Jiang, X.; Gao, Y. Highly Stretchable and Self-Healable Supercapacitor with Reduced Graphene Oxide Based Fiber Springs. ACS Nano 2017, 11, 2066–2074. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Peng, J.; Li, Y.; Hu, H.; Jiang, L.; Cheng, Q. Use of Synergistic Interactions to Fabricate Strong, Tough, and Conductive Artificial Nacre Based on Graphene Oxide and Chitosan. ACS Nano 2015, 9, 9830–9836. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shen, Q.; Zhang, X.; Pan, H.; Lu, Y. Graphene Oxide-Filled Multilayer Coating to Improve Flame-Retardant and Smoke Suppression Properties of Flexible Polyurethane Foam. J. Mater. Sci. 2016, 51, 10361–10374. [Google Scholar] [CrossRef]
- Kreta, A.; Swillam, M.A. Designing of an AFM Cell for In Situ Nanoscopic Study of Electrochemical Deposition. Eng. Proc. 2023, 56, 310. [Google Scholar] [CrossRef]
- Kreta, A.; Gaberšček, M.; Muševič, I. Time-resolved in Situ Electrochemical Atomic Force Microscopy Imaging of the Corrosion Dynamics of AA2024-T3 Using a New Design of Cell. J. Mater. Res. 2021, 36, 79–93. [Google Scholar] [CrossRef]
- Surca, A.K.; Kreta, A.; Mihelčič, M.; Gaberscek, M.; Rodošek, M. Benefits of Coupling of Electrochemical Technique with Either IR, Raman or AFM Technique in the Corrosion Investigation. ECS Meet. Abstr. 2017, MA2017-01, 939. [Google Scholar] [CrossRef]
- Kreta, A.; Hočevar, S.B. An In Situ AFM Study of Electrochemical Bismuth Film Deposition on a Glassy Carbon Substrate Electrode Using a Low Concentration of Bismuth Ions. Eng. Proc. 2023, 31, 27. [Google Scholar]
- Kreta, A.; Swillam, M.A.; Guirguis, A.; Hassanien, A. Unbundling SWCNT Mechanically via Nanomanipulation Using AFM. Eng. Proc. 2023, 56, 83. [Google Scholar] [CrossRef]
- Surca, A.K.; Rodošek, M.; Kreta, A.; Mihelčič, M.; Gaberšček, M. In Situ and Ex Situ Electrochemical Measurements: Spectroelectrochemistry and Atomic Force Microscopy. Hybrid. Org.-Inorg. Interfaces 2017, 793–837. [Google Scholar] [CrossRef]
- Yasakau, K. Application of AFM-Based Techniques in Studies of Corrosion and Corrosion Inhibition of Metallic Alloys. Corros. Mater. Degrad. 2020, 1, 345–372. [Google Scholar] [CrossRef]
- Chen, H.; Qin, Z.; He, M.; Liu, Y.; Wu, Z. Application of Electrochemical Atomic Force Microscopy (EC-AFM) in the Corrosion Study of Metallic Materials. Materials 2020, 13, 668. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, J.; Fullea, J.; Andrade, C.; Gaitero, J.J.; Porro, A. AFM Study of the Early Corrosion of a High Strength Steel in a Diluted Sodium Chloride Solution. Corros. Sci. 2008, 50, 1820. [Google Scholar] [CrossRef]
- Orlikowski, J.; Jazdzewska, A.; Mazur, R.; Darowicki, K. Determination of Pitting Corrosion Stage of Stainless Steel by Galvanodynamic Impedance Spectroscopy. Electrochim. Acta 2017, 253, 403. [Google Scholar] [CrossRef]
- Kreta, A.; Mulec, J.; Oarga-Mulec, A.; Pavlica, E. Raman Detection of Algae on a Silica Substrate. Proceedings 2024, 104, 23. [Google Scholar] [CrossRef]
- Henrik-Klemens, Å.; Bengtsson, F.; Björdal, C.G. Raman Spectroscopic Investigation of Iron-Tannin Precipitates in Waterlogged Archaeological Oak. Stud. Conserv. 2022, 67, 237–247. [Google Scholar] [CrossRef]
- Hurley, B.L.; Qiu, S.; Buchheit, R.G. Raman Spectroscopy Characterization of Aqueous Vanadate Species Interaction with Aluminum Alloy 2024-T3 Surfaces. J. Electrochem. Soc. 2011, 158, C125. [Google Scholar] [CrossRef][Green Version]
- Kreta, A.; Rodošek, M.; Slemenik Perše, L.; Orel, B.; Gaberšček, M.; Šurca Vuk, A. In Situ Electrochemical AFM, Ex Situ IR Reflection-Absorption and Confocal Raman Studies of Corrosion Processes of AA 2024-T3. Corros. Sci. 2016, 104, 290–309. [Google Scholar] [CrossRef]
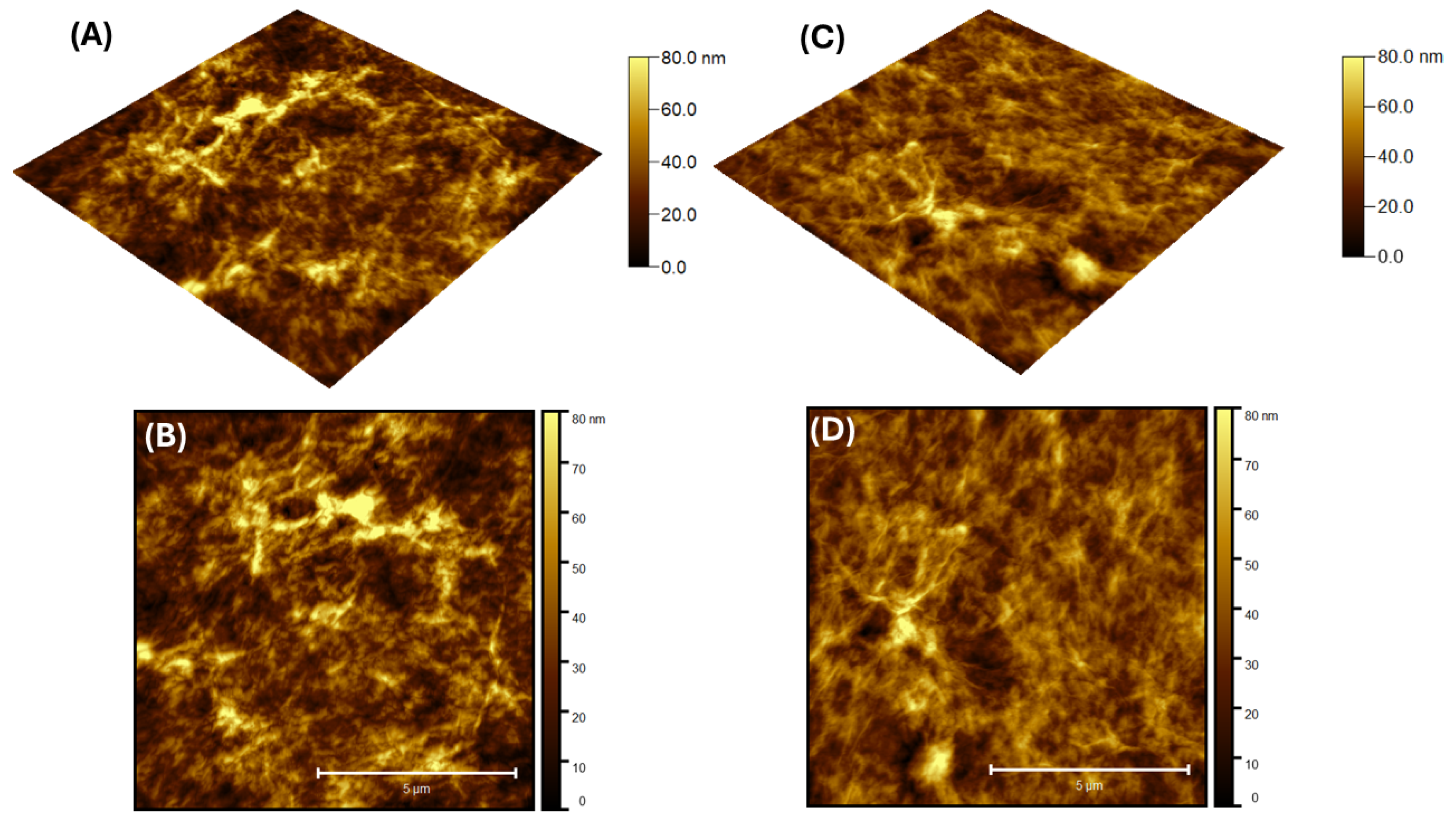
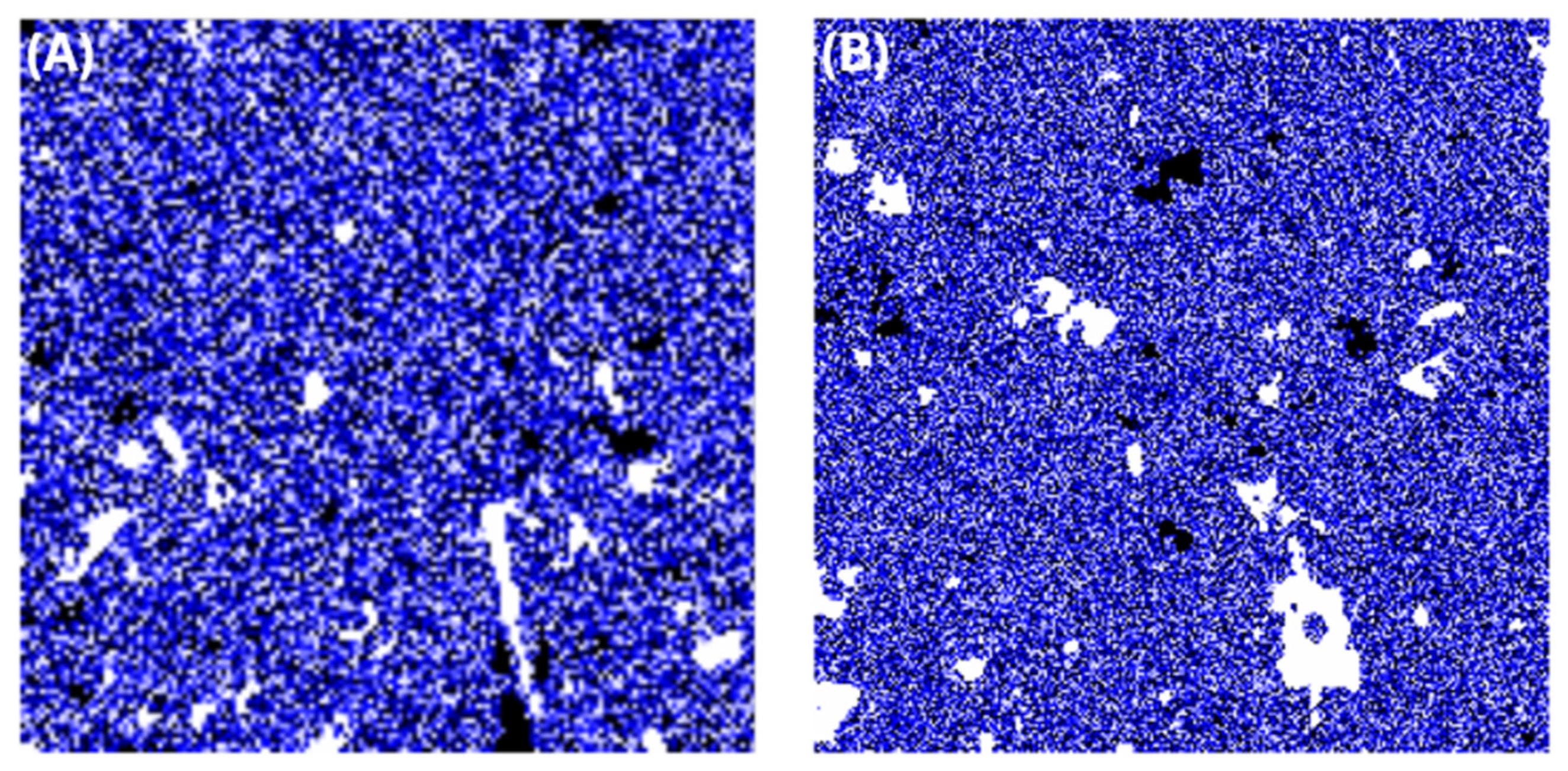
| Maximum Height (nm) | RMS Roughness (nm) | Skewness | |
|---|---|---|---|
| GO | 129 ± 5 | 14.7 ± 4 | 1.05 ± 0.1 |
| RGO | 94 ± 3 | 10.6 ± 2 | 0.49 ± 0.1 |
| R1 (Ohm) | Q1 (F.sa1−1) | a1 | R2 (Ohm) | Q3 (F.sa3−1) | a3 | R3 (Ohm) | |
|---|---|---|---|---|---|---|---|
| RGO | 92.6 | 5.65 × 10−4 | 0.86 | 1326 | 4.29 × 10−5 | 0.2063 | 11.55 |
| GO | 53.43 | 4.20 × 10−4 | 0.87 | 2215 | 2.41 × 10−2 | 1 | 64.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kreta, A.; Jerman, I.; Bele, M.; Surca, A.K.; Gaberšček, M.; Muševič, I. Nanoscopic Characterization of Reduced Graphene Oxide for Anticorrosion Coating of AA2024. Eng. Proc. 2025, 87, 82. https://doi.org/10.3390/engproc2025087082
Kreta A, Jerman I, Bele M, Surca AK, Gaberšček M, Muševič I. Nanoscopic Characterization of Reduced Graphene Oxide for Anticorrosion Coating of AA2024. Engineering Proceedings. 2025; 87(1):82. https://doi.org/10.3390/engproc2025087082
Chicago/Turabian StyleKreta, Ahmed, Ivan Jerman, Marjan Bele, Angelja Kjara Surca, Miran Gaberšček, and Igor Muševič. 2025. "Nanoscopic Characterization of Reduced Graphene Oxide for Anticorrosion Coating of AA2024" Engineering Proceedings 87, no. 1: 82. https://doi.org/10.3390/engproc2025087082
APA StyleKreta, A., Jerman, I., Bele, M., Surca, A. K., Gaberšček, M., & Muševič, I. (2025). Nanoscopic Characterization of Reduced Graphene Oxide for Anticorrosion Coating of AA2024. Engineering Proceedings, 87(1), 82. https://doi.org/10.3390/engproc2025087082







