Abstract
Mangiferin, a polyphenol derived from Mangifera indica, exhibits a wide range of pharmacological activities, positioning it as a promising candidate for applications in medicine. Its potential as an alternative to antibiotics is especially significant in agriculture and medicine, addressing the global demand for non-traditional antibacterial agents. However, its poor water solubility and low bioavailability limit its therapeutic use. Polymeric matrices could be one of the potential approaches to solve these problems, enhancing mangiferin’s bioavailability. Each delivery system exhibits unique properties such as controlled release, prolonged action and organ-specific targeting, in an ideal case each directed to specific diseases and administration routes. While promising in vitro and in vivo results highlight their therapeutic potential, challenges remain in the optimization of the matrix formulations for precise applications. Further research is needed to develop these systems and confirm their efficacy in clinical practice.
1. Introduction
Mangiferin is a biologically active substance, a natural polyphenol extracted from the Mangifera indica plant. Mangiferin possesses antiviral, antitumour, anti-inflammatory and other properties, and it can potentially be used in medicine as an effective drug. The properties of mangiferin are shown in Figure 1.
Mangiferin is also interesting as an alternative antibacterial agent. The use of antibiotics in agriculture is limited or restricted worldwide [1,2,3]. Such measures are being taken in India [1], the United States [2] and the European Union [3]. Due to its unique properties and absence of toxicity, mangiferin has the potential to replace traditional drugs.
However, one of the problems in the use of mangiferin is its poor water solubility (0.36 mM) and low oral bioavailability (1.2%) [4]. Drug delivery matrices could be one of the potential solutions to these problems. Drug delivery systems have been found to enhance drug bioavailability and stability and to attenuate side effects. A wide variety of delivery systems with mangiferin have been developed in recent years [5,6,7]. For example, several types of gold nanoparticles have been developed that deliver mangiferin along with other drugs for cancer therapy [5]. Systems based on natural and synthetic polymers, such as sol-gels and emulsions, have also been investigated [6]. In addition, lipid-based nanosystems have been developed for the transdermal delivery of mangiferin: transferosomes, liposomes and phospholipid vehicles [7].
Developed nanosystems include polymer nanospheres, lipid nanoparticles, self-assembled protein particles and other matrices. Each of them has its own properties and can be applied against a specific disease and for a specific way of administering mangiferin into the body. Some matrices have prolonged action, while some release the drug immediately.
The collection of existing data about drug delivery systems will facilitate their study and enable the development of new matrices with controlled properties. This brief review covers information about the main systems utilizing mangiferin and their properties.
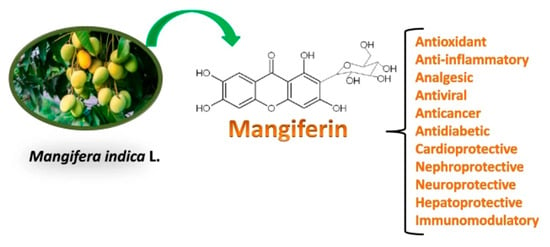
Figure 1.
Some properties of mangiferin [8]. Distributed under the terms of the Creative Commons CC BY license, 2022 Springer.
2. Mangiferin Delivery Systems
2.1. Polymeric Nanoparticles
Biocompatible polymers are widely used materials for the development of drug delivery matrices. They are non-toxic and biodegradable, as well as chemically and mechanically stable; they change their properties with the changing of environmental conditions. Therefore, a lot of work has been undertaken to develop polymeric particles with mangiferin.
Anan Athipornchai et al. fabricated polymer nanospheres composed of a core of carrageenan with mangiferin surrounded by a chitosan shell. At 25 °C, the spheres with the highest mangiferin content (10% by weight of carrageenan) released up to 90% mangiferin within 2 h. The nanospheres had moderate antibacterial activity, but they were harmless to eukaryotic cells (mouse subcutaneous connective tissue cell lines (L929) and a kidney epithelial cell line derived from the African green monkey (Vero cell)) in the in vitro test. The authors developed the nanospheres for seafood preservation, but they believe the spheres could also be used as drug delivery systems [9]. However, in order to use these nanospheres for seafood preservation, more work is needed to find out how mangiferin affects the flavour of the products and how the antibacterial activity of the nanospheres changes in a cold water medium.
Francisco Fabian Razura-Carmona et al. developed poly(lactic-co-glycolic acid)-based mangiferin nanoparticles to inhibit cancer cell proliferation. The particles were synthesised by solvent evaporation, and their average size was 176.7 nm in diameter. It was found that some samples were able to release mangiferin in a controlled manner under acidic environmental conditions. The authors believe that this could potentially be used for drug release in the stomach. However, it was also found that the particles were non-toxic to HepG2 (hepatocellular human carcinoma) and BEAS-2B cells (human lung epithelial cells). Thus, the anti-cancer effect expected by the authors was not detected. More investigations are needed in this area to find out why mangiferin does not exhibit anticancer properties when delivered by this system [10].
Ritu Jain et al. developed polymeric nanoparticles with mangiferin. The matrices were based on folic acid, poly(lactic-co-glycolic acid) (PLGA) and adipic dihydrazide (ADH). The matrices smoothly released more than 98% of mangiferin within 48 h. In an in vivo biodistribution experiment, it was found that the nanoparticles gradually increased the amount of drug in the blood when administered intravenously, reaching a peak at 8 h after the administration. In the in vitro experiment, it was also found that, at the maximum concentration (100 μg/mL), the nanoparticles with mangiferin could effectively inhibit the growth of A549 cells (human lung carcinoma) [11]. In this field, studies of the efficacy and safety of mangiferin delivery systems on in vivo models of various diseases are also required.
José Roberto R. de Souza et al. encapsulated mangiferin in natural polymers using a spray drying technique. The polymers used were pumpkin pectin, citric pectin and chitosan in different combinations, and the emulsifier was Tween 80. Spray particles with a composition of pumpkin pectin/mangiferin/Tween 80 showed the highest encapsulation efficiency. In addition, the particles of different formulations had different average diameters, and the larger the diameter results in the greater the encapsulation efficiency [12]. The mechanisms of interaction between chitosan and the surfactant Tween 80 are discussed in this paper. To confirm their assumptions, the researchers need to conduct further studies with other polymers that have a charge.
Haojue Wang et al. synthesized nanoparticles with an average diameter of 138 nm based on hyaluronic acid, mangiferin and methotrexate. Hyaluronic acid and methotrexate acted as receptor ligands, and mangiferin acted as an anti-inflammatory agent. In the in vitro study, a combination of substances was taken up by k7 cells (mouse osteosarcoma) significantly more strongly than each substance alone. It was also found that the particles significantly reduced the survival rate of k7 cancer cells and activated their apoptosis. At the same time, normal MC3T3-E1 cells (mouse osteoblast) were not as aggressively affected by the particles. This could potentially be used in targeted cancer therapy [13]. However, further development in this area requires research to test the efficacy and safety of this delivery system in in vivo models.
In our research group, we developed nanofibers with mangiferin based on hyaluronic acid. It was found that increasing the concentration of mangiferin led to an increase in the average diameter of fibers up to 291.3 nm at the ratio of mangiferin:hyaluronic acid of 1:5. It was also found in the in vitro experiment that the majority of mangiferin (up to 85–95%) was rapidly released within 10–15 min at pH//// medium, and then the rest of the substance was released gradually through up to 1 h. Such delivery systems could potentially be used in the development of prolonged dosage forms [14].
There are also several works demonstrating the possibility of the use of proteins as the basis of matrices. For example, Rohini Samadarsi et al. synthesised nanoparticles based on β-lactoglobulin, the major whey protein of milk, by a desolvation technique for oral delivery. The encapsulation efficiency was 85–88% depending on the crosslinker used. It was found that nanoparticles aggregated in an acidic medium (pH 4.5 and below). The release of mangiferin from the matrices was negligible at pH 1.2 and increased up to 80% in neutral medium and pH 4.5. These nanoparticles were developed to deliver mangiferin to the colon, so these release results were considered successful by the authors. In addition, the nanoparticles were found to be harmless to probiotic strains of bacteria and toxic to pathogenic strains [15]. In the future, it is possible to study this delivery system in animal models in vivo and then in humans.
From recent work, Pratik Chakraborty et al. developed polymeric nanoparticles with mangiferin based on poly(lactic-co-glycolic) acid with the addition of vitamin E-TPGS. The average diameter of the obtained particles was 162.5 nm, and the encapsulation efficiency of mangiferin was 55.9%. The polymeric particles showed good stability; their properties did not change when stored for 90 days at 4 °C. In an in vitro experiment, it was found that the particles effectively inhibited the viability of MDA-MB-231 (triple-negative breast cancer) cells: the IC50 value of the particles was 3.5 times lower than that of mangiferin powder. In an in vivo experiment in mice, the particles also showed significant anti-cancer properties. However, the authors did not investigate why the value of encapsulation efficiency was so small. This was probably due to the mangiferin:polymer ratio, as well as the interaction between the mangiferin molecule and the polymer molecules. Further research is required in this area [16].
2.2. Nanoparticles Based on Lipids
Lipids are another widely used substance for the creation of delivery matrices. They are also biocompatible, effectively encapsulate mangiferin and have high bioavailability.
Mohamad Allaw et al. developed transferosomes to increase the bioavailability of mangiferin. The transferosomes were based on glycerol and propylene glycol, and mucin was also added to the solution before ultrasonic disintegration. The particles had a significant antioxidant effect on 3T3 fibroblasts (mouse embryonic fibroblasts) in vitro. In vivo, the transferosomes promoted faster wound healing after chemical burn injury [17]. The authors suggest that propylene glycol acts as an enhancer of transferosomes penetration into tissues. To confirm this suggestion and to find other possible enhancers, further research in this area is required.
Rajneet Kaur Khurana et al. developed lipid carriers of mangiferin in a complex with Phospholipon 90 G. The complex was synthesized by the solvent-evaporation method. It was found that the content of mangiferin in a complex with phospholipid was more than 95% in all the samples. The solubility of the complex in water was 25 times higher compared to pure mangiferin. The complex was then used to synthesize nano-lipid carriers by hot emulsification and ultrasonication methods. The lipid carriers in the in vitro experiment showed a prolonged release of mangiferin. Also, lipid carriers were practically harmless for Caco-2 cells (human colorectal adenocarcinoma); within 3 days, the cell survival rate did not fall below 95%. The authors believe that this technology will potentially overcome the difficulties of oral administration of mangiferin [18]. Future development of this delivery system also requires work that examines the stability of the nanocomplexes and their potential use after long-term storage.
Ahmed I. Foudah et al. synthesised solid lipid nanoparticles with mangiferin using a microemulsion technique and ultrasound. Labrafil M 2130 CS was chosen as the solid lipid and Tween 80 as the surfactant. The synthesized spheres had a size from 38.97 to 181.42 nm in diameter and had a high capture efficiency of mangiferin, with a larger particle size leading to a higher capture efficiency. The nanoparticles had significant antioxidant effects comparable to ascorbic acid, and the in vivo experiments in mice also showed strong antidiabetic properties. In addition, it was found that encapsulation in nanoparticles enhanced the properties of mangiferin and allowed it to penetrate deeper into tissues [19]. A study in animal models would examine the safety of this system as well as confirm its efficacy in the treatment of diabetes.
There has also been research into the development of organ-specific systems. Debora Santonocito et al. created mangiferin nanospheres based on Miglyol 812 and Compritol 888 ATO for eye treatment. The size of the nanospheres was 149 nm, and the encapsulation efficiency was about 92%. The authors did not report an in vivo eye study, but they conducted an in vitro toxicity study of the nanospheres on fertilized chicken eggs. The nanospheres were found to be non-toxic and tissue-safe, and the resulting antioxidant activity was higher than that of pure mangiferin. The authors suggest that the particles can potentially be used in the form of drops to treat eye diseases [20].
2.3. Micelles and Self-Assembled Particles
Self-assembled particles based on proteins, polymers and lipids are a promising type of delivery system, as they have high permeability into biological tissues and are also able to protect the drug molecule before it reaches the target area.
Micelles are now being investigated as delivery systems. Rajneet Kaur Khurana et al. developed self-assembled micelles with the inclusion of mangiferin and synthetic vitamin E (E TPGS). The micelles were based on Phospholipid 90 G and polyethylene glycol 200. Particles were obtained with diameters ranging from 15 to 60 nm, and a high release rate of mangiferin was observed: more than 80% within 15 min and more than 90% within 1 h. In the in vivo experiment, the micelles showed increased bioavailability and uptake by cancer cell lines (MCF-7 and MDA-MB-231) compared to pure mangiferin. In the ex vivo experiment on rat intestines, micelles showed significantly higher bioavailability compared to free mangiferin [21].
Mengdi Wang et al. synthesised nanoparticles with mangiferin for diabetes control. These were self-assembled polyethylene glycol-polycaprolactone-based nanoparticles. Glucagon-like peptide 1 was immobilized on these particles, which acted as the receptor in targeting drug delivery to pancreatic β-cells. In an in vivo study, it was found that the nanoparticles had a better therapeutic effect compared to a similar amount of pure mangiferin. The particles were able to control blood glucose levels, inhibited apoptosis and stimulated pancreatic β-cell proliferation [22]. The overall study design is summarized in Figure 2.
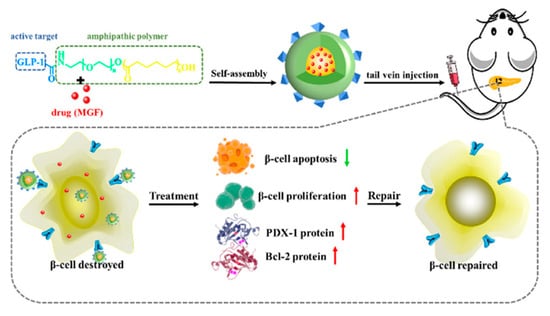
Figure 2.
Polyethylene glycol-polycaprolactone-based nanoparticles for diabetes control [22]. Reprinted (adapted) with permission from Wang, M.; Zhang, Z.; Huo, Q.; Wang, M.; Sun, Y.; Liu, H.; Chang, J.; He, B.; Liang, Y. Targeted Polymeric Nanoparticles Based on Mangiferin for Enhanced Protection of Pancreatic β-Cells and Type 1 Diabetes Mellitus Efficacy. ACS Appl Mater Interfaces 2022, 14, 11092–11103, doi:10.1021/acsami.1c22964. Copyright 2022 American Chemical Society.
2.4. Gels and Emulsions
Gels and emulsions are promising systems for use in surgery and wound dressings, as they create a favourable environment for tissue regeneration and repair.
Athit Pipattanawarothai et al. created a series of gels with mangiferin. The composition of the gels included combinations of polyvinyl alcohol, chitosan, gelatin and cross-linker tetraethoxysilane. In an in vitro experiment on the release of mangiferin from matrices at pH 5.5 and pH 10.0, the hydrogel with a composition of 90% polyvinyl alcohol, 5% gelatin and 5% chitosan showed the best result. When this gel was studied with a cross-linker under the same conditions, it was found that tetraethoxysilane improved the release kinetics at pH 10.0 but had a negative effect at pH 5.5. The authors considered these results to be positive, since an alkaline environment is observed in wounds, and the developed gels will potentially be used in wound dressings. In addition, it was found that gelatin weakens the intermolecular interaction between mangiferin and polyvinyl alcohol–chitosan complex, so it increases the amount of mangiferin released from the matrix [23].
Cui Meng et al. synthesised a protein-based matrix. They used the self-assembled peptide RADA16-I. The average particle size was 492 nm. By adding the suspension of nanoparticles in PBS, a hydrogel was prepared. Mangiferin was released from the matrices intensively within 6 h, then released smoothly for up to 48 h. Moreover, the higher the concentration of mangiferin in the hydrogel was, the less mangiferin was released into the medium. The hydrogel was also found to have time- and dose-dependent cytotoxicity towards the KYSE 30 (human esophageal carcinoma) and DLD-1 (human colon adenocarcinoma) cell lines. However, the hydrogel was virtually harmless to healthy 293T cells (human kidney epithelial) and was even less toxic than pure mangiferin. The authors consider the nanoparticles promising for applications in several types of surgery [24].
There are known attempts to develop gels and emulsions for drug delivery. María Pleguezuelos-Villa et al. created hyaluronic acid-based nanoemulsions with mangiferin. A release experiment revealed that nanoemulsions without hyaluronic acid released mangiferin relatively quickly (up to 10% overnight). Nanoemulsions with hyaluronic acid released mangiferin slowly, and the presence of Transcutol-P solvent in the emulsion had a stronger effect than the molecular weight of hyaluronic acid. Thus, the kinetics of drug release can be regulated by adjusting the composition of the emulsion. In an in vivo experiment on mice, the emulsions promoted active skin healing after chemical burns [25].
Recently, Neungreuthai Chomchoei et al. developed an electrospray-mangiferin nanoparticle gel to protect the skin from the harmful effects of sunlight and slow down the aging process. They first synthesized nanoparticles by electrospraying a cellulose acetate solution and then incorporated these particles with mangiferin into a gel based on Carbopol® ultraz 21. The gel had high sun protection properties as well as antioxidant activity. Such gel has the potential to be an effective skin protection agent, but work is required in this area to investigate the safety of the formulations not only for humans but also for marine organisms, as the gel could potentially be washed from human skin into the environment [26].
2.5. Polymer Films
Films are also one of the matrix types under investigation. Films are not difficult to manufacture and can release drugs efficiently due to their large surface area.
In some studies, films are manufactured for oral administration. For example, Hebat-Allah S. Tohamy et al. manufactured edible films with mangiferin based on hydroxyethyl cellulose. It was found that mangiferin significantly affected the mechanical properties of the films. As the concentration of mangiferin increased, the porosity of the films first decreased and then increased almost to a maximum at a mangiferin content of 10%. In addition, the researchers believe that increasing the concentration of mangiferin in the films impaired water uptake by the films. In the in vitro experiment, the Gram-positive bacteria were selectively inhibited [27].
Rungkan Boonnattakorn et al. incorporated mangiferin into an ethylene vinyl acetate-based polymer matrix and investigated the effect of the vinyl acetate content on the properties of the matrix. It was found that matrices with 40% vinyl acetate content released significantly more mangiferin (up to 80%) than matrices with 12, 18 and 25% vinyl acetate content. In addition, the authors found that the release of mangiferin was linearly dependent on the vinyl acetate content of the matrix in the range of 18–40%. The authors believe that this observation was due to crystallinity and dissolution effects. As the vinyl acetate content increased, the dissolution of mangiferin in the polymer matrix increased, hence, the release rate increased [28].
In the paper [29], the authors investigated the effect of the vinyl acetate content of ethylene vinyl acetate on the properties of ethylene vinyl acetate films with mangiferin in the presence of surfactants. It was found that in the presence of the surfactants Span®20 and Pluronic®P123, mangiferin dissolved better in the polymer matrix, and mangiferin accumulation was lower compared to films without surfactants. At the same time, Span®20 promoted less mangiferin accumulation than Pluronic®P123. The authors also partially confirmed their findings from their previous work on film properties [29].
The data presented in Table 1 summarize the known mangiferin delivery matrices with the indication of mangiferin concentrations used and the type of cells lines studied.

Table 1.
Some mangiferin delivery matrices and the type of cells studied.
3. Conclusions and Future Perspectives
Newly developed mangiferin delivery systems have the potential to become potential drugs against bacterial infections, inflammation and cancer. The matrices have significant encapsulating efficiency; also, they significantly increase the bioavailability of mangiferin. Mangiferin in matrices can effectively inhibit the growth of cancer cells and pathogenic bacteria. However, there are still several challenges in this field. Matrices can affect the properties of mangiferin, reducing its effectiveness or altering its effects. In addition, more research is needed in this field to develop precise matrix formulations that will be suitable for each specific treatment method and target. The specific organ, route of administration and disease: these three factors need to be considered when designing matrices. Also, additional studies on in vivo models are required to clarify the effects of the matrices and its toxicity.
The chemical interaction between mangiferin and matrix material has not received sufficient attention in the mentioned works even though this interaction is one of the key factors in the development of prolonged-acting and controlled-release systems. In addition, few authors have developed a delivery system with a targeting action, despite the fact that such matrix modification can significantly enhance the therapeutic effects. Finally, another important area of research in this field is the development of delivery systems for mangiferin together with other drugs. Such combinations can potentially compensate for the disadvantages of each drug separately.
If the described ideas are be realized, mangiferin delivery matrices could become an effective tool in the therapy of many diseases.
Author Contributions
Conceptualization, S.N.M. and P.P.S.; methodology, S.N.M. and P.P.S.; validation, V.I.K.; formal analysis, R.O.S.; investigation, R.O.S.; resources, P.P.S.; data curation, P.P.S.; writing—original draft preparation, R.O.S. and V.I.K.; writing—review and editing, S.N.M. and P.P.S.; visualization, R.O.S. and V.I.K.; supervision, S.N.M. and P.P.S.; project administration, P.P.S.; funding acquisition, P.P.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation, project number 24-23-00269. Link to information about the project: https://rscf.ru/en/project/24-23-00269/. Accessed on 4 June 2025.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dutta, T.K.; Yadav, S.K.; Chatterjee, A. Antibiotics as Feed Additives for Livestock: Human Health Concerns. Indian J. Anim. Health 2019, 58, 121. [Google Scholar] [CrossRef]
- Casey, J.A.; Tartof, S.Y.; Davis, M.F.; Nachman, K.E.; Price, L.; Liu, C.; Yu, K.; Gupta, V.; Innes, G.K.; Tseng, H.F.; et al. Impact of a Statewide Livestock Antibiotic Use Policy on Resistance in Human Urine Escherichia Coli Isolates: A Synthetic Control Analysis. Environ. Health Perspect. 2023, 131, 27007. [Google Scholar] [CrossRef]
- Simjee, S.; Ippolito, G. European Regulations on Prevention Use of Antimicrobials from January 2022. Braz. J. Vet. Med. 2022, 44, e000822. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, H.; Moon, Y.; Kwak, S.; Kang, C.G.; Park, C.; Jo, J.; Kim, S.W.; Pal, K.; Kang, D.H.; et al. Enhancement of the Water Solubility and Antioxidant Capacities of Mangiferin by Transglucosylation Using a Cyclodextrin Glycosyltransferase. Enzyme Microb. Technol. 2022, 159, 110065. [Google Scholar] [CrossRef]
- Sarfraz, M.; Khan, A.; Batiha, G.E.S.; Akhtar, M.F.; Saleem, A.; Ajiboye, B.O.; Kamal, M.; Ali, A.; Alotaibi, N.M.; Aaghaz, S.; et al. Nanotechnology-Based Drug Delivery Approaches of Mangiferin: Promises, Reality and Challenges in Cancer Chemotherapy. Cancers 2023, 15, 4194. [Google Scholar] [CrossRef]
- Morozkina, S.N.; Nhung Vu, T.H.; Generalova, Y.E.; Snetkov, P.P.; Uspenskaya, M.V. Mangiferin as New Potential Anti-Cancer Agent and Mangiferin-Integrated Polymer Systems—A Novel Research Direction. Biomolecules 2021, 11, 79. [Google Scholar] [CrossRef]
- Baghel, M.; Baghel, I.; Kumari, P.; Bharkatiya, M.; Joshi, G.; Sakure, K.; Badwaik, H. Nano-Delivery Systems and Therapeutic Applications of Phytodrug Mangiferin. Appl. Biochem. Biotechnol. 2024, 196, 7429–7463. [Google Scholar] [CrossRef]
- Barakat, S.; Nasr, M.; Ahmed, R.F.; Badawy, S.; Mortada, N. Recent Formulation Advances of Mangiferin. Rev. Bras. Farmacogn. 2022, 32, 871–882. [Google Scholar] [CrossRef]
- Athipornchai, A.; Pabunrueang, P.; Trakulsujaritchok, T. Mangiferin Loaded Carrageenan/Chitosan Core-Shell Hydrogel Beads: Preparation, Characterization and Proposed Application. Food Hydrocoll. 2024, 147, 109394. [Google Scholar] [CrossRef]
- Razura-Carmona, F.F.; Pérez-Larios, A.; González-Silva, N.; Herrera-Martínez, M.; Medina-Torres, L.; Sáyago-Ayerdi, S.G.; Sánchez-Burgos, J.A. Mangiferin-Loaded Polymeric Nanoparticles: Optical Characterization, Effect of Antitopoisomerase I, and Cytotoxicity. Cancers 2019, 11, 1965. [Google Scholar] [CrossRef]
- Jain, R.; Tiwari, R.; Agrawal, O.P.; Shukla, A.K. Bioinspired Folic Acid-ADH-PLGA Polymeric Nanoparticles Loaded with Mangiferin and Piperine: A Promising Strategy for Targeted Delivery in Multidrug-Resistant Lung Cancer Cells. Asian J. Chem. 2023, 35, 2640–2650. [Google Scholar] [CrossRef]
- de Souza, J.R.R.; Feitosa, J.P.A.; Ricardo, N.M.P.S.; Trevisan, M.T.S.; de Paula, H.C.B.; Ulrich, C.M.; Owen, R.W. Spray-Drying Encapsulation of Mangiferin Using Natural Polymers. Food Hydrocoll. 2013, 33, 10–18. [Google Scholar] [CrossRef]
- Wang, H.; Shao, W.; Lu, X.; Gao, C.; Fang, L.; Yang, X.; Zhu, P. Synthesis, Characterization, and in Vitro Anti-Tumor Activity Studies of the Hyaluronic Acid-Mangiferin-Methotrexate Nanodrug Targeted Delivery System. Int. J. Biol. Macromol. 2023, 239, 124208. [Google Scholar] [CrossRef]
- Shaikenov, R.; Klimshina, V.; Generalova, Y.; Serbun, P.; Kosova, A.; Dorogov, M.; Morozkina, S.; Snetkov, P. Electrospun Hyaluronan-Based Nanofibers with Mangiferin: Preparation, Morphology, and Drug Release Kinetics. In Proceedings of the IOCBE 2024, Basel, Switzerland, 28 November 2024; p. 2. [Google Scholar]
- Samadarsi, R.; Dutta, D. Design and Characterization of Mangiferin Nanoparticles for Oral Delivery. J. Food Eng. 2019, 247, 80–94. [Google Scholar] [CrossRef]
- Chakraborty, P.; Das, A.; Chatterjee, S.; Bairagi, A.; Bhattacharya, H.; Bhattacharyya, C.; Chatterjee, N.; Sil, P.C.; Dewanjee, S. Formulation and Evaluation of Polymeric Nanoparticles to Improve in Vivo Chemotherapeutic Efficacy of Mangiferin against Breast Cancer. Naunyn Schmiedebergs Arch. Pharmacol. 2025. [Google Scholar] [CrossRef]
- Allaw, M.; Pleguezuelos-Villa, M.; Manca, M.L.; Caddeo, C.; Aroffu, M.; Nacher, A.; Diez-Sales, O.; Saurí, A.R.; Ferrer, E.E.; Fadda, A.M.; et al. Innovative Strategies to Treat Skin Wounds with Mangiferin: Fabrication of Transfersomes Modified with Glycols and Mucin. Nanomedicine 2020, 15, 1671–1685. [Google Scholar] [CrossRef]
- Khurana, R.K.; Bansal, A.K.; Beg, S.; Burrow, A.J.; Katare, O.P.; Singh, K.K.; Singh, B. Enhancing Biopharmaceutical Attributes of Phospholipid Complex-Loaded Nanostructured Lipidic Carriers of Mangiferin: Systematic Development, Characterization and Evaluation. Int. J. Pharm. 2017, 518, 289–306. [Google Scholar] [CrossRef]
- Foudah, A.I.; Ayman Salkini, M.; Alqarni, M.H.; Alam, A. Preparation and Evaluation of Antidiabetic Activity of Mangiferin-Loaded Solid Lipid Nanoparticles. Saudi J. Biol. Sci. 2024, 31, 103946. [Google Scholar] [CrossRef]
- Santonocito, D.; Vivero-Lopez, M.; Lauro, M.R.; Torrisi, C.; Castelli, F.; Sarpietro, M.G.; Puglia, C. Design of Nanotechnological Carriers for Ocular Delivery of Mangiferin: Preformulation Study. Molecules 2022, 27, 1328. [Google Scholar] [CrossRef]
- Khurana, R.K.; Gaspar, B.L.; Welsby, G.; Katare, O.P.; Singh, K.K.; Singh, B. Improving the Biopharmaceutical Attributes of Mangiferin Using Vitami E-TPGS Co-Loaded Self-Assembled Phosholipidic Nano-Mixed Micellar Systems. Drug Deliv. Transl. Res. 2018, 8, 617–632. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, Z.; Huo, Q.; Wang, M.; Sun, Y.; Liu, H.; Chang, J.; He, B.; Liang, Y. Targeted Polymeric Nanoparticles Based on Mangiferin for Enhanced Protection of Pancreatic β-Cells and Type 1 Diabetes Mellitus Efficacy. ACS Appl. Mater. Interfaces 2022, 14, 11092–11103. [Google Scholar] [CrossRef] [PubMed]
- Pipattanawarothai, A.; Athipornchai, A.; Sripreechasak, P.; Trakulsujaritchok, T. Development of Polymeric Hydrogels for Potential Biomedical Applications. Burapha Sci. J. 2019, 24, 885–900. [Google Scholar]
- Meng, C.; Wei, W.; Wang, Y.; Zhang, K.; Zhang, T.; Tang, Y.; Tang, F. Study of the Interaction between Self-Assembling Peptide and Mangiferin and in Vitro Release of Mangiferin from in Situ Hydrogel. Int. J. Nanomedicine 2019, 14, 7447–7460. [Google Scholar] [CrossRef] [PubMed]
- Pleguezuelos-Villa, M.; Nácher, A.; Hernández, M.J.; Ofelia Vila Buso, M.A.; Ruiz Sauri, A.; Díez-Sales, O. Mangiferin Nanoemulsions in Treatment of Inflammatory Disorders and Skin Regeneration. Int. J. Pharm. 2019, 564, 299–307. [Google Scholar] [CrossRef]
- Chomchoei, N.; Leelapornpisid, P.; Tipduangta, P.; Sirithunyalug, J.; Sirithunyalug, B.; Samutrtai, P. Electrospray-Mangiferin Nanoparticles Gel: A Promising Agent for Sun and Age Defense. Cosmetics 2024, 11, 93. [Google Scholar] [CrossRef]
- Tohamy, H.A.S.; El-Sakhawy, M.; El-Masry, H.M.; Saleh, I.A.; AbdelMohsen, M.M. Preparation of Hydroxyethyl Cellulose/ Mangiferin Edible Films and Their Antimicrobial Properties. BMC Chem. 2022, 16, 113. [Google Scholar] [CrossRef]
- Boonnattakorn, R.; Chonhenchob, V.; Siddiq, M.; Singh, S.P. Controlled Release of Mangiferin Using Ethylene Vinyl Acetate Matrix for Antioxidant Packaging. Packag. Technol. Sci. 2015, 28, 241–252. [Google Scholar] [CrossRef]
- Boonnattakorn, R.; Sane, A.; Chonhenchob, V. Antioxidant Microemulsion-Based Ethylene Vinyl Acetate Film Containing Mangiferin and Surfactants. MATEC Web Conf. 2016, 67, 6101. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).