Electrospinning Poly(acrylonitrile) (PAN) Nanofiber Mats with Mushroom Mycelium Powder †
Abstract
1. Introduction
2. Materials and Methods
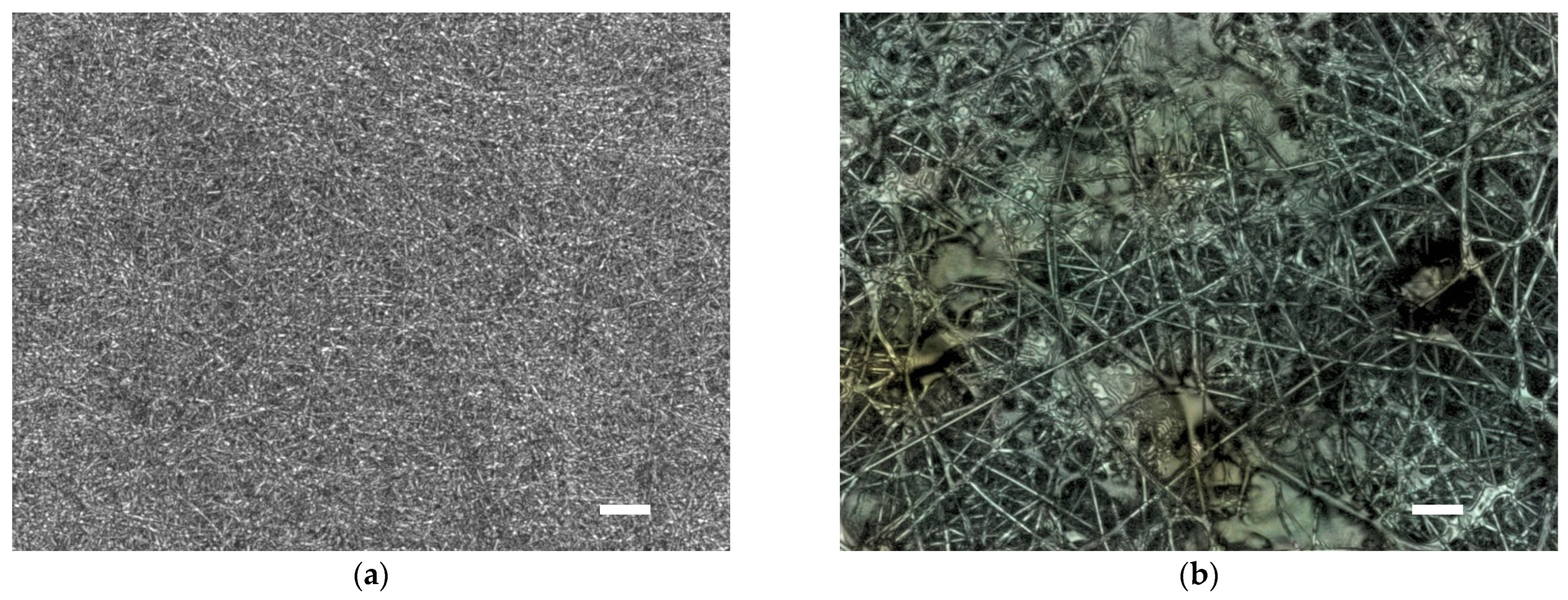
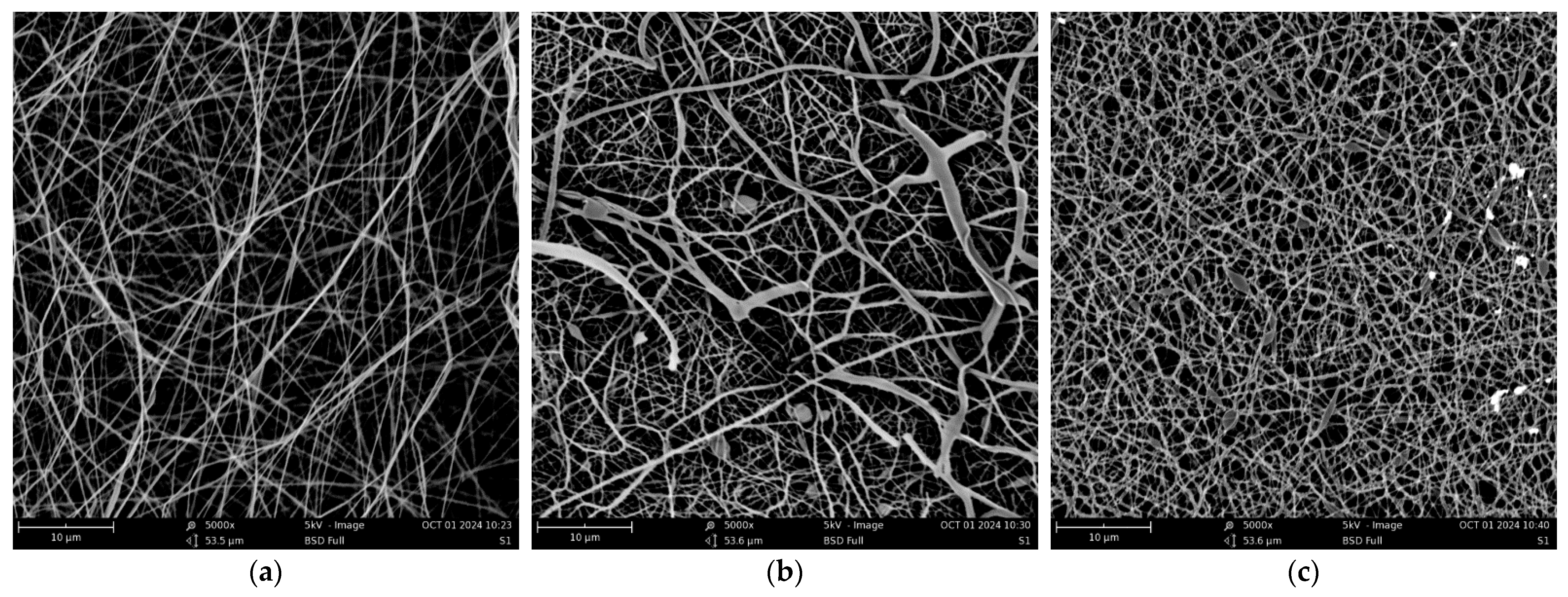
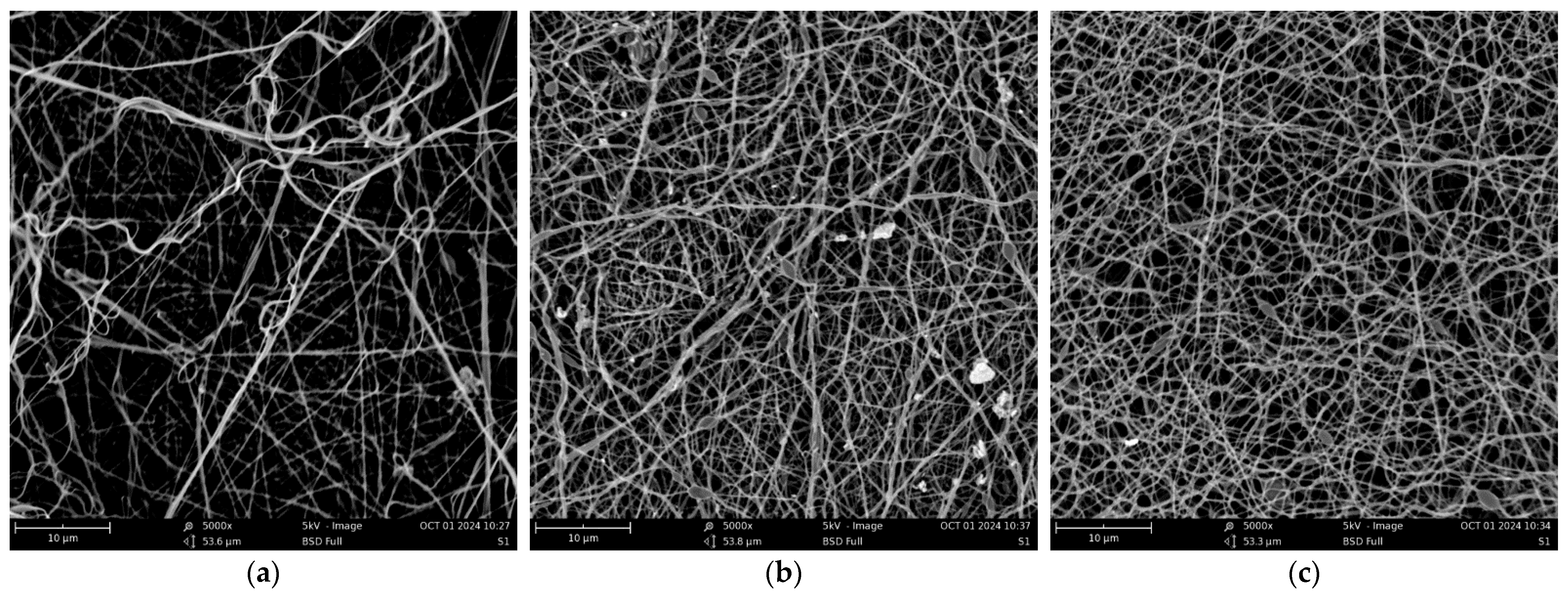
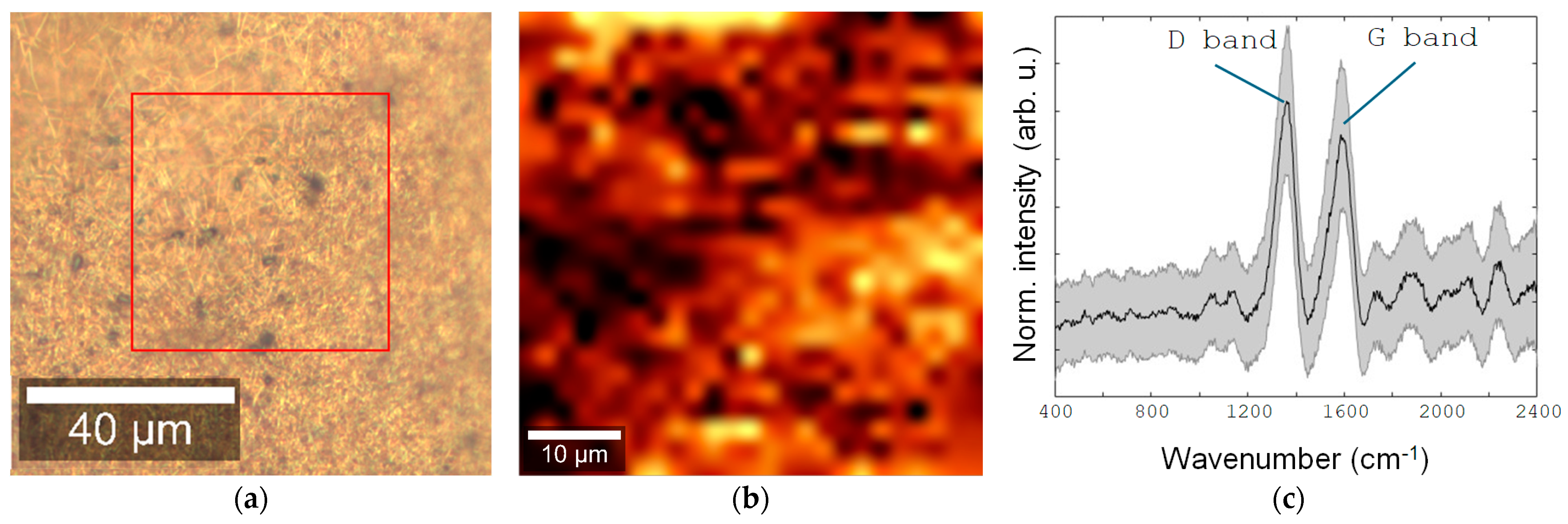
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PAN | Poly(acrylonitrile) |
References
- Adeli-Sardou, M.; Yaghoobi, M.M.; Torkzadeh-Mahani, M.; Dodel, M. Controlled release of lawsone from polycaprolactone/gelatin electrospun nanofibers for skin tissue regeneration. Int. J. Biol. Macromol. 2019, 124, 478–491. [Google Scholar] [CrossRef] [PubMed]
- Yeo, M.; Ki, G.H. Anisotropically Aligned Cell-Laden Nanofibrous Bundle Fabricated via Cell Electrospinning to Regenerate Skeletal Muscle Tissue. Small 2018, 14, e1803491. [Google Scholar] [CrossRef] [PubMed]
- Roche, R.; Yalcinkaya, F. Electrospun Polyacrylonitrile Nanofibrous Membranes for Point-of-Use Water and Air Cleaning. ChemistryOpen 2019, 8, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Zhou, M.J.; Zhang, H.N.; Quan, Z.Z.; Wang, R.W.; Yin, X.H. Sandwich-structures fibrous membranes with low filtration resistance for effective PM2.5 capture via one-step needleless electrospinning. Mater. Res. Express 2019, 6, 035027. [Google Scholar] [CrossRef]
- Yalcinkaya, F. A review on advanced nanofiber technology for membrane distillation. J. Eng. Fiber. Fabr. 2019, 14, 1558925018824901. [Google Scholar] [CrossRef]
- Rasouli, R.; Barhoum, A.; Bechelany, M.; Dufresne, A. Nanofibers for Biomedical and Healthcare Applications. Macromol. Biosci. 2019, 19, e1800256. [Google Scholar] [CrossRef]
- Horne, J.; McLoughlin, L.; Bridgers, B.; Wujcik, E.K. Recent developments in nanofiber-based sensors for disease detection, immunosensing, and monitoring. Sens. Actuators Rep. 2020, 2, 100005. [Google Scholar] [CrossRef]
- Chen, X.W.; Li, H.; Xu, Z.T.; Lu, L.J.; Pan, Z.F.; Mao, Y.C. Electrospun Nanofiber-Based Bioinspired Artificial Skins for Healthcare Monitoring and Human-Machine Interaction. Biomimetics 2023, 8, 223. [Google Scholar] [CrossRef]
- Mei, L.; Ren, Y.M.; Gu, Y.C.; Li, X.L.; Wang, C.; Du, Y.; Fan, R.R.; Gao, X.; Chen, H.F.; Tong, A.P.; et al. Strengthened and Thermally Resistant Poly(lactic acid)-Based Composite Nanofibers Prepared via Easy Stereocomplexation with Antibacterial Effects. ACS Appl. Mater. Inter. 2018, 10, 42992–43002. [Google Scholar] [CrossRef]
- Reis, F.S.; Pereira, E.; Barros, L.; Sousa, M.J.; Martins, A.; Ferreira, I.C.F.R. Biomolecule profiles in inedible wild mushrooms with antioxidant value. Molecules 2011, 16, 4328–4338. [Google Scholar] [CrossRef]
- Mishra, V.; Tomar, S.; Yadav, P.; Vishwakarma, S.; Singh, M.P. Elemental Analysis, Phytochemical Screening and Evaluation of Antioxidant, Antibacterial and Anticancer Activity of Pleurotus ostreatus through In Vitro and In Silico Approaches. Metabolites 2022, 19, 821. [Google Scholar] [CrossRef] [PubMed]
- Andrjc, D.C.; Knez, Z.; Marevci, M.K. Antioxidant, antibacterial, antitumor, antifungal, antiviral, anti-inflammatory, and nevro-protective activity of Ganoderma lucidum: An overview. Front. Pharmacol. 2022, 13, 934982. [Google Scholar]
- Qiu, Z.H.; Wu, X.L.; Gao, W.; Zhang, J.X.; Huang, C.Y. High temperature induced disruption of the cell wall integrity and structure in Pleurotus ostreatus mycelia. Appl. Microbiol. Biotechnol. 2018, 102, 6627–6636. [Google Scholar] [CrossRef] [PubMed]
- Serag, E.; Eltawila, A.M.; Salem, E.M.; El-Maghraby, A.; Abd El-Aziz, A.M. Development of an innovative cylindrical carbon nanofiber/gelatin-polycaprolactone hydrogel scaffold for enhanced bone regeneration. Int. J. Biol. Macromol. 2025, 306, 141250. [Google Scholar] [CrossRef]
- Trabelsi, M.; Mamun, A.; Klöcker, M.; Sabantina, L.; Großerhode, C.; Blachowicz, T.; Ehrmann, A. Increased Mechanical Properties of Carbon Nanofiber Mats for Possible Medical Applications. Fibers 2019, 7, 98. [Google Scholar] [CrossRef]
- Brockhagen, B.; Hellert, C.; Grothe, T.; Güth, U.; Storck, J.L.; Ehrmann, A.; Wortmann, M. Freestanding Flexible Carbon Nanofiber Mats for Energy Storage Applications. Mater. Proc. 2025, 21, 1. [Google Scholar] [CrossRef]
- Wehlage, D.; Blattner, H.; Mamun, A.; Kutzli, I.; Diestelhorst, E.; Rattenholl, A.; Gudermann, F.; Lütkemeyer, D.; Ehrmann, A. Cell growth on electrospun nanofiber mats from polyacrylonitrile (PAN) blends. AIMS Bioeng. 2020, 7, 43–54. [Google Scholar] [CrossRef]
- Wehlage, D.; Blattner, H.; Sabantina, L.; Böttjer, R.; Grothe, T.; Rattenholl, A.; Gudermann, F.; Lütkemeyer, D.; Ehrmann, A. Sterilization of PAN/Gelatine Nanofibrous Mats for Cell Growth. Tekstilec 2019, 62, 78–88. [Google Scholar] [CrossRef]
- Barbosa, H.F.G.; Francisco, D.S.; Ferreira, A.P.G.; Cavalheiro, É.T.G. A new look towards the thermal decomposition of chitins and chitosans with different degrees of deacetylation by coupled TG-FTIR. Carbohydr. Polym. 2019, 225, 115232. [Google Scholar] [CrossRef]
- Storck, J.L.; Hellert, C.; Brockhagen, B.; Wortmann, M.; Diestelhorst, E.; Frese, N.; Grothe, T.; Ehrmann, A. Metallic Supports Accelerate Carbonization and Improve Morphological Stability of Polyacrylonitrile Nanofibers during Heat Treatment. Materials 2021, 14, 4686. [Google Scholar] [CrossRef]
- Sabantina, L.; Böttjer, R.; Wehlage, D.; Grothe, T.; Klöcker, M.; García-Mateos, F.; Rodríguez-Mirasol, J.; Cordero, T.; Ehrmann, A. Morphological study of stabilization and carbonization of polyacrylonitrile/TiO2 nanofiber mats. J. Eng. Fibers Fabr. 2019, 14, 1558925019862242. [Google Scholar] [CrossRef]
- Yang, H.P.; Yan, R.; Chen, H.P.; Lee, D.H.; Zheng, C.G. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Escalante, J.; Chen, W.-H.; Tabatabaei, M.S.; Hoang, A.T.; Kwon, E.E.; Lin, Y.-Y.A.; Saravanakumar, A. Pyrolysis of lignocellulosic, algal, plastic, and other biomass wastes for biofuel production and circular bioeconomy: A review of thermogravimetric analysis (TGA) approach. Renew. Sustain. Energy Rev. 2022, 169, 112914. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mpofu, N.S.; Stepula, E.; Güth, U.; Ehrmann, A.; Sabantina, L. Electrospinning Poly(acrylonitrile) (PAN) Nanofiber Mats with Mushroom Mycelium Powder. Eng. Proc. 2025, 87, 45. https://doi.org/10.3390/engproc2025087045
Mpofu NS, Stepula E, Güth U, Ehrmann A, Sabantina L. Electrospinning Poly(acrylonitrile) (PAN) Nanofiber Mats with Mushroom Mycelium Powder. Engineering Proceedings. 2025; 87(1):45. https://doi.org/10.3390/engproc2025087045
Chicago/Turabian StyleMpofu, Nonsikelelo Sheron, Elzbieta Stepula, Uwe Güth, Andrea Ehrmann, and Lilia Sabantina. 2025. "Electrospinning Poly(acrylonitrile) (PAN) Nanofiber Mats with Mushroom Mycelium Powder" Engineering Proceedings 87, no. 1: 45. https://doi.org/10.3390/engproc2025087045
APA StyleMpofu, N. S., Stepula, E., Güth, U., Ehrmann, A., & Sabantina, L. (2025). Electrospinning Poly(acrylonitrile) (PAN) Nanofiber Mats with Mushroom Mycelium Powder. Engineering Proceedings, 87(1), 45. https://doi.org/10.3390/engproc2025087045







