Analyzing the Thermal Behavior and Phase Transitions of ZnSnO3 Prepared via Chemical Precipitation †
Abstract
1. Introduction
2. Experimental
3. Results and Discussion
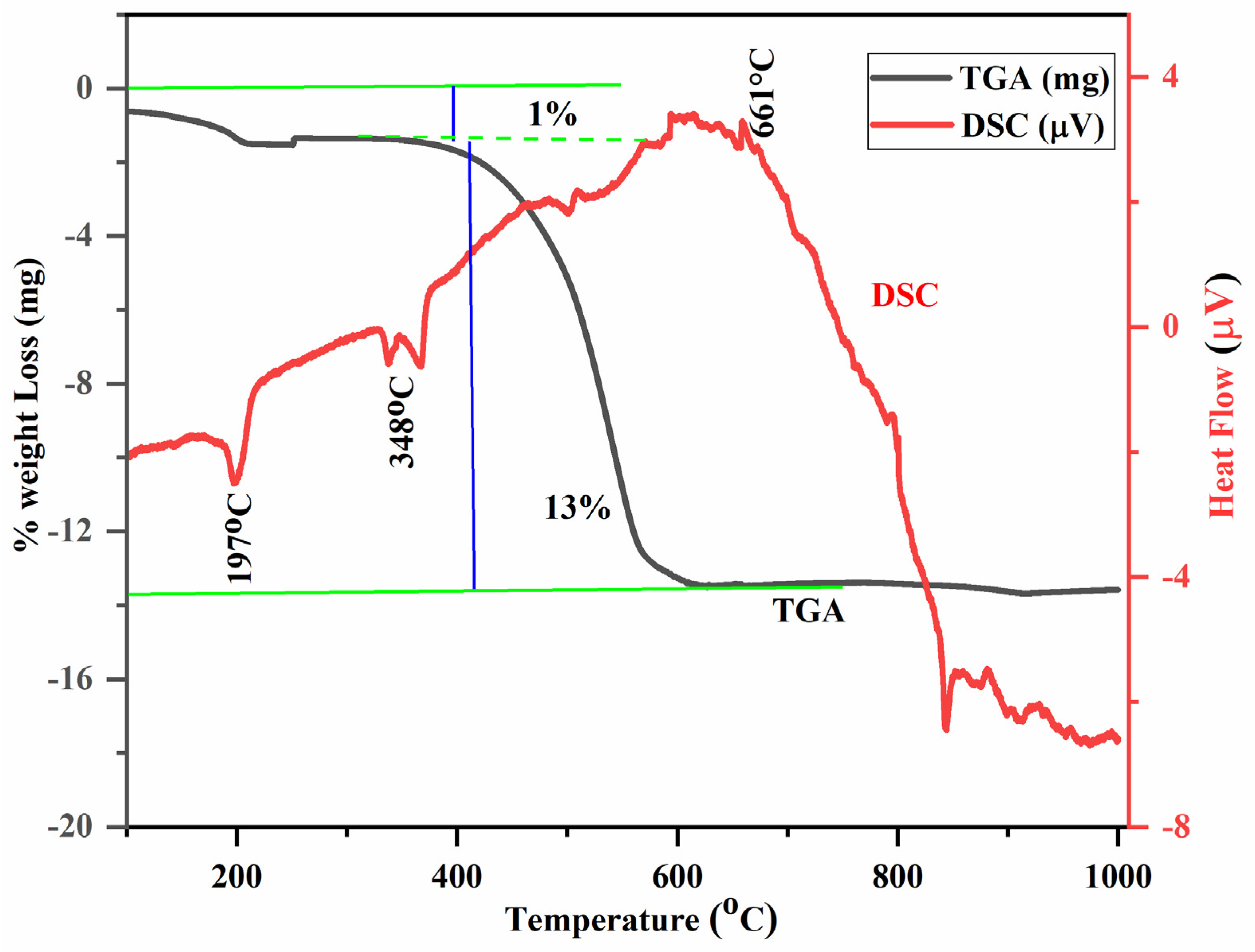
3.1. TGA–DSC Analysis
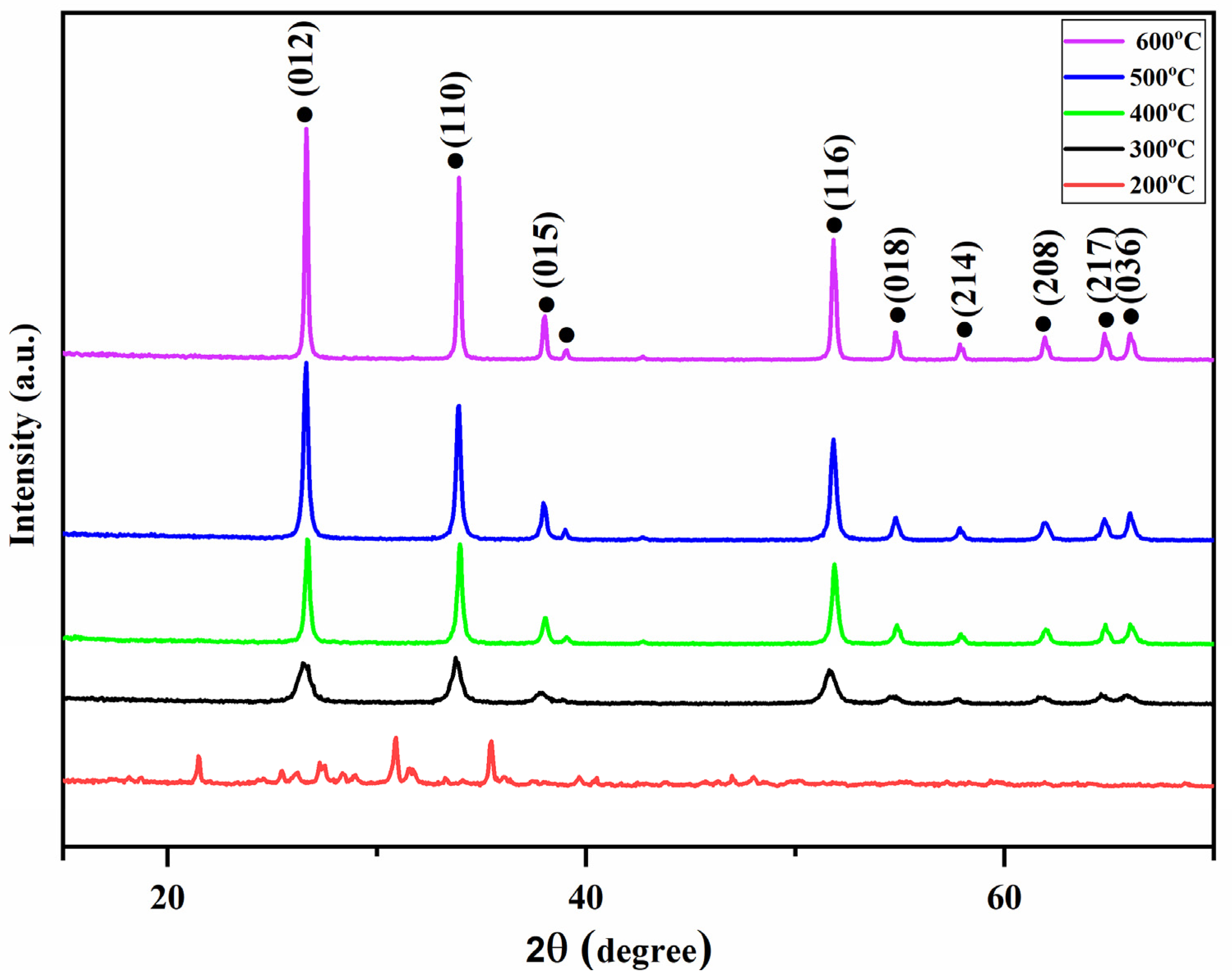
3.2. Structural Analysis
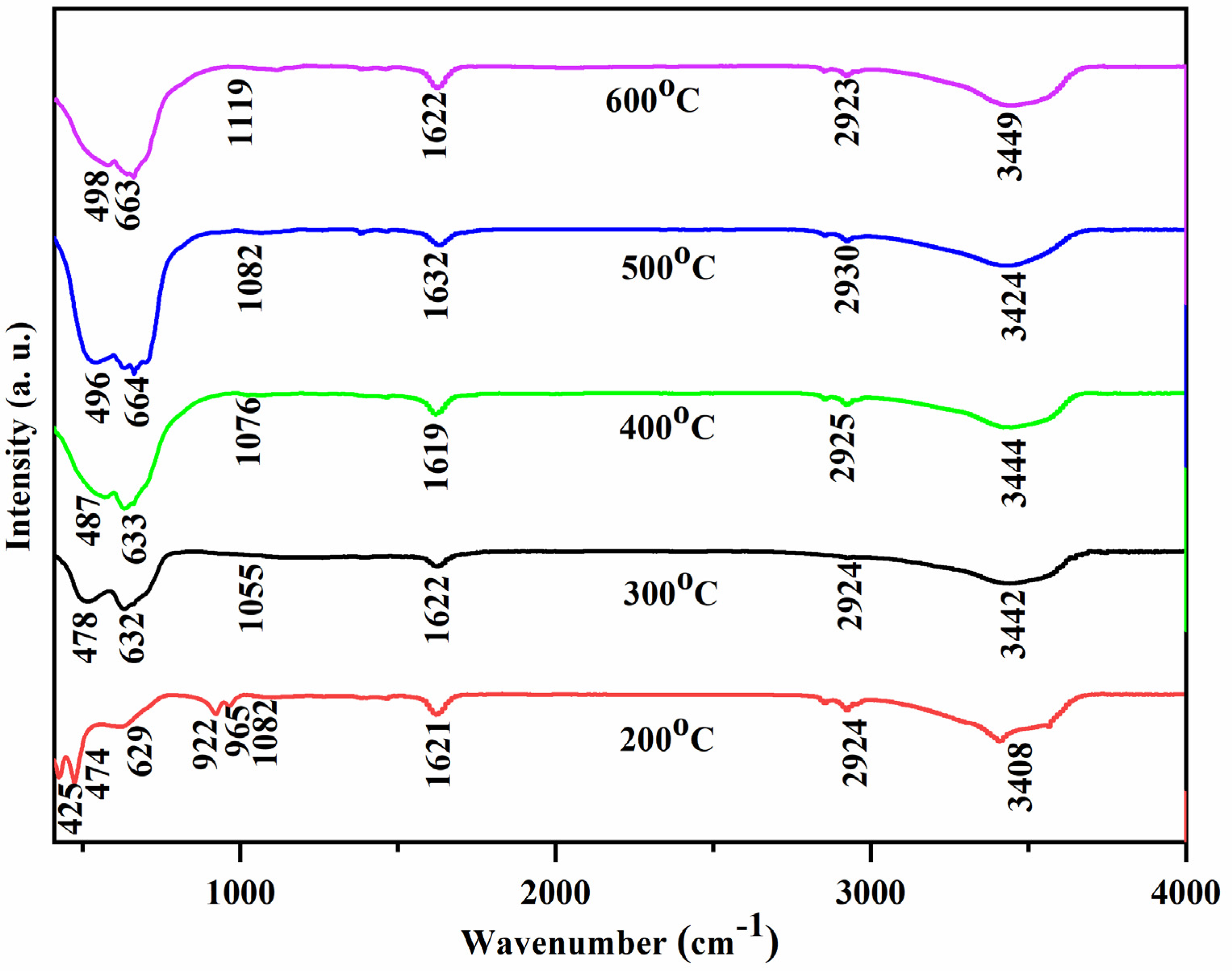
3.3. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
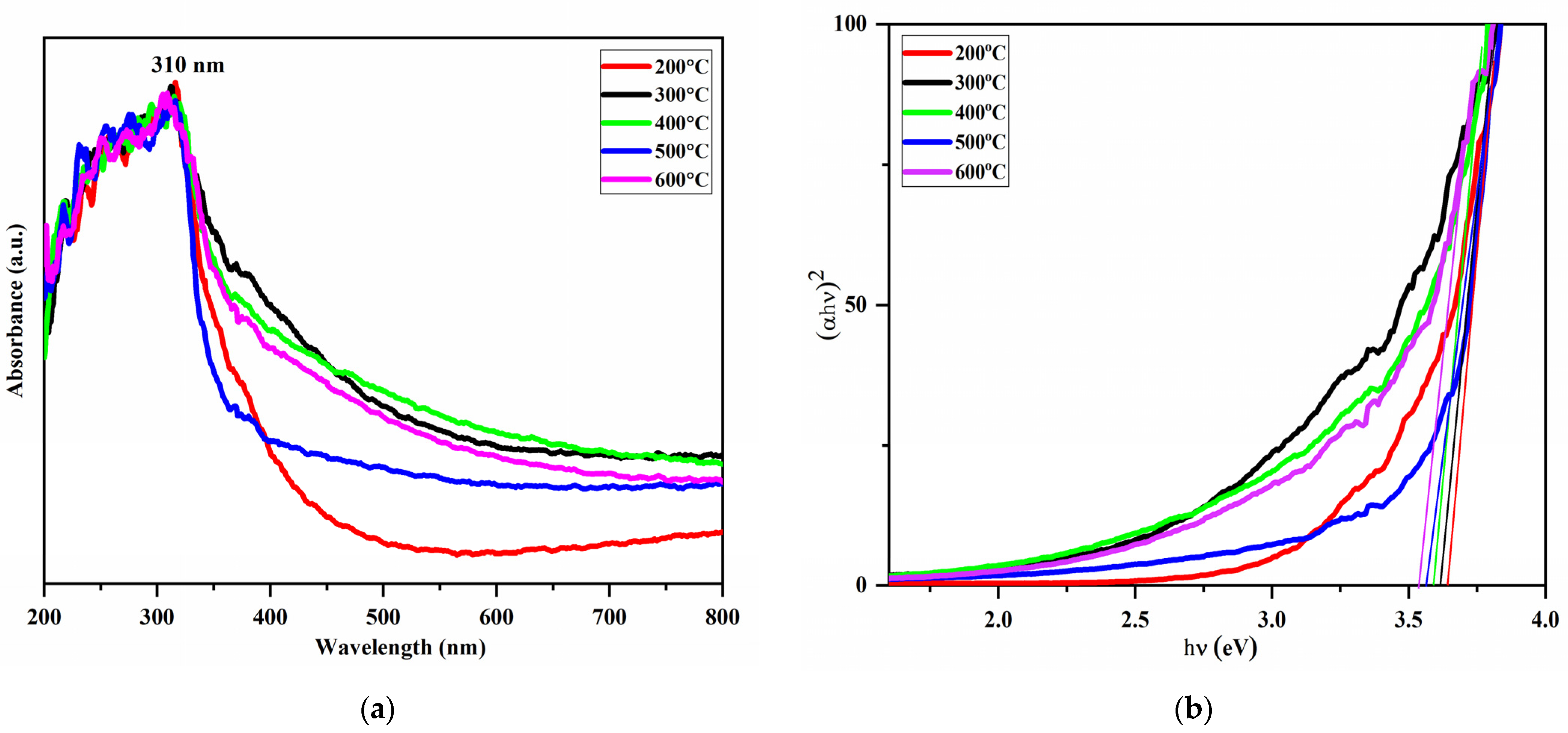
3.4. Uv-Vis Spectroscopy Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El Radaf, I.M. Promising novel transparent conductive F-doped ZnSnO3 thin films for optoelectronic applications. J. Mater. Sci. Mater. Electron. 2023, 34, 215. [Google Scholar] [CrossRef]
- Ashiq, M.G.B.; Mahmood, Q.; Haq, B.U.; Flemban, T.H.; Kattan, N.A.; Alshahrani, T.; Laref, A. The study of electronics, optoelectronics, thermoelectric, and mechanical properties of Zn/CdSnO3 perovskites. Mater. Sci. Semicond. Process. 2022, 137, 106229. [Google Scholar] [CrossRef]
- Taha, T.A.M.; Alshammari, A.H.; Sanad, M.M.; Elsherif, S.S.; Eraky, M.S.; Alenad, A.M.; El-Nasser, K.S.; Hamdeh, H.H. Impact of Ca2+, Ce3+ codoping on ZnSnO3-SnO2 heterostructure for dielectric, optoelectronic and solar cell applications. J. Electroanal. Chem. 2024, 952, 117955. [Google Scholar] [CrossRef]
- Sim, C.K.; Majid, S.R.; Mahmood, N.Z. ZnSnO3/mesoporous biocarbon composite towards sustainable electrode material for energy storage device. Microchem. J. 2021, 164, 105968. [Google Scholar] [CrossRef]
- Zhai, Y.; Wei, W.; Hu, H.; Jing, J.; Lv, X.; Xu, Y.; Xie, J. Reduced graphene oxide decorated CoSnO3@ ZnSnO3 with multi-component double-layered hollow nanoboxes for high energy storage and capacity retention asymmetric supercapacitors. J. Alloys Compd. 2021, 857, 157536. [Google Scholar] [CrossRef]
- Song, P.; Wang, Q.; Yang, Z. Biomorphic synthesis of ZnSnO3 hollow fibers for gas sensing application. Sens. Actuators B Chem. 2011, 156, 983–989. [Google Scholar] [CrossRef]
- Huang, J.; Xu, X.; Gu, C.; Wang, W.; Geng, B.; Sun, Y.; Liu, J. Size-controlled synthesis of porous ZnSnO3 cubes and their gas-sensing and photocatalysis properties. Sens. Actuators B Chem. 2012, 171, 572–579. [Google Scholar] [CrossRef]
- Wang, X.; Xia, M.; Li, H.; Zhu, X.; Leng, B.; Tao, T.; Xu, W.; Xu, J. Preparation of transparent amorphous ZnSnO3 cubic nanoparticles and light-induced homostructures: Application in UV sensor and room-temperature gas sensor. Appl. Surf. Sci. 2019, 493, 862–872. [Google Scholar] [CrossRef]
- Yin, Y.; Shen, Y.; Zhou, P.; Lu, R.; Li, A.; Zhao, S.; Liu, W.; Wei, D.; Wei, K. Fabrication, characterization and n-propanol sensing properties of perovskite-type ZnSnO3 nanospheres based gas sensor. Appl. Surf. Sci. 2020, 509, 145335. [Google Scholar] [CrossRef]
- Guo, W. Hollow and porous ZnSnO3 gas sensor for ethanol gas detection. J. Electrochem. Soc. 2016, 163, B131. [Google Scholar] [CrossRef]
- Guo, R.; Tian, R.; Shi, D.; Li, H.; Liu, H. S-Doped ZnSnO3 nanoparticles with narrow band gaps for photocatalytic wastewater treatment. ACS Appl. Nano Mater. 2019, 2, 7755–7765. [Google Scholar] [CrossRef]
- Rehman, Z.U.; Rehman, S.U.; Bilal, M.; Butt, F.K.; Hussain, A.; Jrar, J.A.; Zheng, K.; Zhang, Y.; Xu, X.; Wang, X.; et al. Engineering of interfacial electric field by g-C3N4/ZnSnO3 heterojunction for excellent photocatalytic applications. J. Clean. Prod. 2024, 469, 143258. [Google Scholar] [CrossRef]
- Md Sin, N.D.; Mamat, M.H.; Aziz, A.; Rusop, M. Fabrication of Humidity Sensor deposited by sol-gel immersion method of nano-cubic structured ZnSnO3/ZnO based thin film. Int. J. Electr. Electron. Syst. Res. (IEESR) 2013, 6, 11–18. [Google Scholar]
- Jia, X.; Tian, M.; Zhang, Z.; Dai, R.; Wu, X.; Song, H. Highly sensitive formaldehyde chemical sensor based on in situ precipitation synthesis of ZnSnO3 microspheres. J. Mater. Sci. Mater. Electron. 2015, 26, 6224–6231. [Google Scholar] [CrossRef]
- Mayedwa, N.; Mongwaketsi, N.; Khamlich, S.; Kaviyarasu, K.; Matinise, N.; Maaza, M. Green Synthesis of Zin Tin Oxide (ZnSnO3) Nanoparticles Using Aspalathus Linearis Natural Extracts: Structural, Morphological, Optical and Electrochemistry Study. Appl. Surf. Sci. 2018, 446, 250–257. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, L.; Li, Q.; Wu, Y.; Li, Q.; Wang, T. An evolution from 3D face-centered-cubic ZnSnO3 nanocubes to 2D orthorhombic ZnSnO3 nanosheets with excellent gas sensing performance. Nanotechnology 2012, 23, 415501. [Google Scholar] [CrossRef]
- Mekprasart, W.; Tangcharoen, T.; Nakhanivej, P.; Pecharapa, W. Synthesis and Characterization of Cubic-like Zinc Stannate Powders Prepared by Co-precipitation Method. Chiang Mai J. Sci. 2016, 43, 345–350. [Google Scholar]
- Lua, L.; Zhang, A.; Xiao, Y.; Gong, F.; Jia, D.; Li, F. Effect of solid inorganic salts on the formation of cubic-like aggregates of ZnSnO3 nanoparticles in solventless, organic-free reactions and their gas sensing behaviors. Mater. Sci. Eng. B 2012, 177, 942–948. [Google Scholar] [CrossRef]
- Chen, Y.; Qu, B.; Mei, L.; Lei, D.; Chen, L.; Li, Q.; Wang, T. Synthesis of ZnSnO3 Mesocrystals from Regular Cube-like to Orthorhombic Sheet-like Structures and Their Comparative Electrochemical Properties in Li-ion Batteries. J. Mater. Chem. 2012, 22, 25373–25379. [Google Scholar] [CrossRef]
- Koppala, S.; Balan, R.; Banerjee, I.; Li, K.; Xu, L.; Liu, H.; Kumar, D.K.; Reddy, K.R.; Sadhu, V. Room temperature synthesis of novel worm-like tin oxide nanoparticles for photocatalytic degradation of organic pollutants. Mater. Sci. Energy Technol. 2021, 4, 113–118. [Google Scholar] [CrossRef]
- Ul Haq, M.; Zhang, Z.; Chen, X.; Rahman, N.; Khan, S.; Khatoon, R.; Hassan, S.S.; Ye, Z.; Zhu, L. A two-step synthesis of microsphere-decorated fibers based on NiO/ZnSnO3 composites towards superior ethanol sensitivity performance. J. Alloys Compd. 2019, 777, 73–83. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, P.; Bao, D.; Wang, L.Q.; Chen, Y.J.; Zhou, X.M.; Yang, P.P.; Sun, S.C.; Zhang, M.L. One Pot, two phases: Individual orthorhombic and face-centered cubic ZnSnO3 obtained synchronously in one solution. Inorg. Chem. 2014, 53, 12289–12296. [Google Scholar] [CrossRef]
- Riahi, I.; Khalfallah, B.; Chaabouni, F. Physico-chemical properties of perovskite ZnSnO3 thin films deposited on glass and silicon wafers by RF magnetron sputtering. Opt. Quant. Electron. 2022, 54, 514. [Google Scholar] [CrossRef]
- Usha, P.; Ramesh, S.; Jayamurugan, P.; Mariappan, R. Temperature Dependence and Potential of Nebulizer Spray Pyrolysis on Zinc Stannate (ZnSnO3) Thin Films for Ammonia Gas Sensors. Sens. Imaging 2024, 25, 18. [Google Scholar] [CrossRef]
- Khatun, M.; Mandal, B.; Mukherjee, S.; Mitra, P. Dependence of the efficiency of ZnSnO3 nanopowder photocatalyst against Congo red on the sintering temperature of the as-prepared samples. J. Mater. Sci. Mater. Electron. 2024, 35, 325. [Google Scholar] [CrossRef]
- Anitha, A.; Ponnusamy, V. Optical and electrochemical studies on single-phase ZnSnO3 nanostructures—A photosensitive approach. Surf. Interfaces 2024, 51, 104747. [Google Scholar] [CrossRef]
- Ibrahim, D.M.; Gaber, A.A.; Reda, A.E.; Aziz, D.A.A.; Ajiba, N.A. Structural, optical, and dielectric properties of sol-gel derived perovskite ZnSnO3 nanomaterials. J. Sol-Gel Sci. Technol. 2024, 112, 703–714. [Google Scholar] [CrossRef]
- Hussen, M.K.; Dejene, F.B. Influence of annealing temperature on material properties of red emitting ZnGa2O4: Cr3+ nanostructures. J. Sol-Gel Sci. Technol. 2018, 88, 454–464. [Google Scholar] [CrossRef]
- Habibi, M.H.; Mardani, M. Synthesis and characterization of bi-component ZnSnO3/Zn2SnO4 (perovskite/spinel) nano-composites for photocatalytic degradation of Intracron Blue: Structural, opto-electronic and morphology study. J. Mol. Liq. 2017, 238, 397–401. [Google Scholar] [CrossRef]
- Choi, H.H.; Siddiqui, G.U.; Yang, B.-S.; Mustafa, M. Synthesis of ZnSnO3 nanocubes and thin film fabrication of (ZnSnO3/PMMA) composite through electrospray deposition. J. Mater. Sci. Mater. Electron. 2015, 26, 5690–5696. [Google Scholar] [CrossRef]
- Dong, S.; Cui, L.; Zhang, W.; Xia, L.; Zhou, S.; Russell, C.K.; Fan, M.; Feng, J.; Sun, J. Double shelled ZnSnO3 hollow cubes for efficient photocatalytic degradation of antibiotic wastewater. Chem. Eng. J. 2019, 384, 123279. [Google Scholar] [CrossRef]
- Lui, Y.; Yang, Z.-H.; Song, P.-P.; Xu, R.; Wang, H. Facile Synthesis of Bi2MoO6/ZnSnO3 Heterojunction with Enhanced Visible Light Photocatalytic Degradation of Methylene Blue. Appl. Surf. Sci. 2018, 430, 561–570. [Google Scholar] [CrossRef]
- Paul, S.; Basak, S.; Ali, W. Zinc Stannate Nanostructure: Is It a New Class of Material for Multifunctional Cotton Textiles? ACS Omega 2019, 4, 21827–21838. [Google Scholar] [CrossRef] [PubMed]
- Kumar, Y.; Shirage, P.M. Highest coercivity and considerable saturation magnetization of CoFe2O4 nanoparticles with tunable band gap prepared by thermal decomposition approach. J. Mater. Sci. 2017, 52, 4840–4851. [Google Scholar] [CrossRef]
- Saafi, I.; Dridi, R.; Mimouni, R.; Amlouk, A.; Yumak, A.; Boubaker, K.; Petkova, P.; Amlouk, M. Microstructural and optical properties of SnO2-ZnSnO3 ceramics. Ceram. Int. 2016, 42, 6273–6381. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Indhumathi, R.; Priya, A.S.; Samyuktha, B.S. Analyzing the Thermal Behavior and Phase Transitions of ZnSnO3 Prepared via Chemical Precipitation. Eng. Proc. 2025, 87, 4. https://doi.org/10.3390/engproc2025087004
Indhumathi R, Priya AS, Samyuktha BS. Analyzing the Thermal Behavior and Phase Transitions of ZnSnO3 Prepared via Chemical Precipitation. Engineering Proceedings. 2025; 87(1):4. https://doi.org/10.3390/engproc2025087004
Chicago/Turabian StyleIndhumathi, Ragupathi, Arumugasamy Sathiya Priya, and Baskar Sumathi Samyuktha. 2025. "Analyzing the Thermal Behavior and Phase Transitions of ZnSnO3 Prepared via Chemical Precipitation" Engineering Proceedings 87, no. 1: 4. https://doi.org/10.3390/engproc2025087004
APA StyleIndhumathi, R., Priya, A. S., & Samyuktha, B. S. (2025). Analyzing the Thermal Behavior and Phase Transitions of ZnSnO3 Prepared via Chemical Precipitation. Engineering Proceedings, 87(1), 4. https://doi.org/10.3390/engproc2025087004





