Abstract
In this study, beans aquafaba was used as the emulsifying agent for oil-in-water (o/w) emulsions with sunflower oil concentrations of 30% and 60%. The primary approach to stabilizing such emulsions is by increasing their viscosity through the incorporation of selected polysaccharides. For this purpose, xanthan gum or pregelatinized corn starch were added as stabilizers. The effects of oil content and different stabilizers on the microstructure and rheological properties were evaluated using laser diffraction and rotational viscometry. A pre-optimized seed-to-water ratio of 1:1.5 yielded beans aquafaba with a protein concentration of 0.5%. Further evaporation was used to increase the protein content to 0.8%. The aquafaba-based emulsion samples exhibited a bimodal particle size distribution. An increase in both oil and xanthan gum content had minimal impact on the mean volume diameter of emulsion particles, whereas the addition of pregelatinized corn starch significantly increased this value. All emulsions exhibited pseudoplastic flow behavior. The flow curves were approximated using the power-law and Herschel–Bulkley models. The calculated dynamic yield shear stresses consistently increased with increasing content of both oil and stabilizer in the range from 0.3 to 5.0 Pa. It is worth noting that in emulsions with an oil content of 30%, the addition of xanthan gum had a significant impact on this indicator, while in emulsions with an oil content of 60%, the addition of pregelatinized corn starch had a greater impact. Consequently, higher concentrations of the selected polysaccharides led to the formation of more viscous systems exhibiting enhanced stability. The developed food emulsions based on beans aquafaba are a promising technology in the development of vegetarian products.
1. Introduction
The increasing scientific and practical interest in alternative protein sources arises from the necessity to expand the range of raw materials that comply with modern ethical, environmental, and religious requirements [1]. Among the promising sources is aquafaba—a liquid exhibiting emulsifying, foaming, thickening, and gel-forming properties, which is produced during the technological process of preparing legume seeds for culinary readiness. Aquafaba reflects the properties and chemical composition of the original legume raw material from which it is derived, albeit with lower intensity. In vitro studies indicate that aquafaba demonstrates antioxidant and anticancer properties [2], attributed to the presence of bioactive peptides and oligosaccharides which diffuse into the aqueous phase during the thermal processing of legume seeds. This has been observed in both aquafaba derived from canned beans and chickpeas, as well as from pressure-cooked chickpea seeds, as reported in study [3]. The diffusion process of water-soluble nutrients determines the functional and technological properties of aquafaba.
Due to the considerable diversity among legumes, which vary in chemical composition and technological properties, there is a scientifically supported need to investigate aquafaba derived from different legumes. This approach will promote the use of alternative raw materials in the food industry and provide a deeper insight into their technological potential. Beans were selected as the research object since the majority of studies to date focus on chickpeas or soybeans aquafaba. This choice was based on publications highlighting the positive physiological effects of beans consumption on human health and their functional–technological properties [4]. Beans are a widely cultivated agricultural crop, offering agroecological advantages and high economic accessibility. These attributes position beans as an important food source. However, to explore their potential as a raw material for aquafaba production, further research is required.
The current study seeks to examine the microstructural and rheological properties of emulsions based on beans aquafaba with varying fat and polysaccharide content. This research direction will enable the optimization of beans aquafaba applications in the food industry, especially in the context of creating stable and functional products based on this crude material. The relevance of the research direction is confirmed by existing publications [5,6,7], where the emulsifying properties of aquafaba have been utilized for the production of food products. However, these studies specifically focus on aquafaba derived from canned beans or freshly cooked chickpea aquafaba.
Expanding the information base on the properties of aquafaba derived from various legumes will contribute to the diversification and resource efficiency of the food industry, as well as to the broadening of its raw material base, which is particularly relevant given the growing plant-based product market. Food market analysts [8] predict that this segment will grow at a compound annual growth rate (CAGR) of 12.2% until 2033.
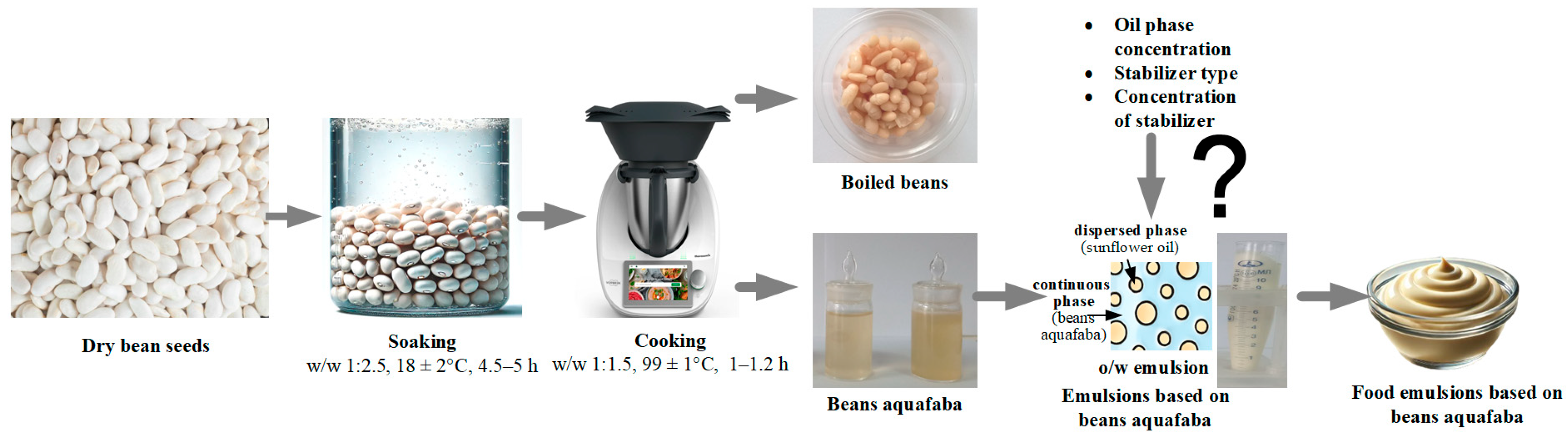
The research is presented in Figure 1.

Figure 1.
Preparation of beans aquafaba.
2. Materials and Methods
2.1. Materials
Beans of the “Mavka” variety from the 2023 harvest, cultivated in the Kyiv region of Ukraine, along with sunflower oil (refined, deodorized, and winterized, containing 0.05–0.06% moisture by weight, branded as OleinaTM, DP Suntrade, Kyiv, Ukraine), were procured from local stores from local suppliers. The beans used in the experiment were stored in a cool, dark, and dry place, away from heat, moisture, and direct sunlight, to ensure their preservation under optimal conditions. The Ziboxan F200 xanthan gum, obtained from Deosen Biochemical Ltd. (Ordos, China), was in a powdered form, with at least 92% of the particles passing through a 200-mesh (75 μm) sieve. Additionally, ULTRA-TEX® 2131 corn starch, a pregelatinized and chemically modified starch derived from waxy maize, was sourced from Ingredion Germany GmbH (Hamburg, Germany). All reagents used in the analysis were of analytical-grade quality.
2.2. Sampling
2.2.1. Preparation of Aquafaba from Beans
The aquafaba was made from beans using the optimized method described as outlined in [9]. The procedure included pre-soaking and subsequent cooking in water, employing either boiling (99 ± 1 °C, the steam pressure was approximately 1 atm (101 ± 1 kPa)) or pressure cooking (119 ± 1 °C, the steam pressure was approximately 2 atm (202 ± 1 kPa)). First, dry bean seeds were sorted to remove damaged specimens and any foreign materials. Approximately 100 g of selected seeds were washed and soaked in potable water at a weight ratio of 1:2.5. This process was carried out at 18 ± 2 °C for 4.5–5 h. After the soaking process, the water was drained and discarded, and the rehydrated seeds (100 g) were rinsed with fresh potable water.
The legumes were cooked using a Thermomix TM6, a semi-automated kitchen appliance (Vorwerk SE & Co. KG, Wuppertal, Germany). The seeds were then mixed with 150 mL of water and cooked at 99 ± 1 °C for 60–80 min. The resulting aquafaba was separated from the cooked seeds with a stainless steel sieve.
The functional and technological properties of aquafaba are largely determined by its dry matter content, particularly the protein content, which acts as a plant-based emulsifier and facilitates the formation of emulsions. The accumulation of dry matter in beans aquafaba differs from other legumes due to the biological characteristics of the seed coat of bean grains, as detailed in [9]. To address this, a boiling method was employed to increase the concentration of dry matter in the beans aquafaba. The aquafaba was boiled until a dry matter content of 4.2 ± 0.2% was achieved. The boiling procedure was carried out using a Thermomix TM6 kitchen appliance (Vorwerk SE & Co. KG, Wuppertal, Germany) at a controlled temperature of 99 ± 1 °C. The prepared aquafaba was then cooled to room temperature and stored in a refrigerator at 2–6 °C.
2.2.2. Emulsion Preparation
The investigated emulsion samples based on beans aquafaba (BAE) were prepared in the same way with two different oil phase concentrations: 30% (BAE30), and 60% (BAE60). Emulsion samples based on aquafaba are classified as oil-in-water (o/w) emulsions.
At the initial stage, the required quantity of oil was added to the aquafaba phase and emulsified (10,000 rpm for 15 min). Next, the prepared emulsions were stabilized by incorporating one of the hydrocolloids at the specified concentrations using a digital homogenizer (IKA ULTRA-TURRAX T 25, IKA, Breisgau, Germany). The component ratios are provided in Table 1. The prepared samples were then stored in a refrigerator at 2–6 °C. The microstructural and rheological properties were measured at least 24 h after the sample preparation.

Table 1.
Composition (%) of emulsion samples based on beans aquafaba (BAE).
The impact of varying stabilizer levels on the microstructural and rheological behavior of emulsion samples was analyzed by incorporating different amounts of stabilizers.
In this study, xanthan gum was used at concentrations of 0.1% or 0.2% w/w, and pregelatinized corn starch was used at 1.0% or 2.0% w/w.
2.3. Methods
Protein content was determined spectrometrically following the method described in [10], using a UV-1200 spectrometer (Shanghai Mapada Instruments Co., Shanghai, China). The UV absorbance was measured at a fixed wavelength of 562 nm. Bovine serum albumin was used as the standard reference. The dry matter content was assessed using a Precisa EM 120-HR moisture analyzer (Gravimetrics AG, Dietikon, Switzerland).
The pH measurements of the samples were conducted three times using a Metrohm 692 pH/Ion meter (Herisau, Switzerland), which was equipped with a Unitrode combined glass electrode featuring Pt1000 technology.
We used a particle size analyzer (PSA 1190, Anton Paar, Graz, Austria) within a measurement range of 0.1–2500 µm, following the method described in [5] without alterations. The analyzer employs optical methods using diffraction analysis based on the Fraunhofer diffraction model. Before measurements, the sample in the diffractometer cell was treated with ultrasound (50 W) for 1 min. To assess particle size, the emulsion was diluted with potable water (~30 °C) at an approximate 1:100 ratio relative to its original concentration to reduce potential multi-scattering effects. The diluted sample was gradually introduced into the measuring cell to ensure the appropriate field-of-view coverage. Measurements were performed at stirrer speeds varying between 150 and 450 rpm. The De Brouckere mean diameter was used to characterize the particle size distribution as the volume-weighted mean diameter D[4,3]. For the determination of the volume-weighted mean diameter (D[4,3]) and SPAN index, we used the formulas specified in ISO 13320:2020 Particle Size Analysis—Laser Diffraction Methods [11].
Aquafaba solutions comprise a suspension with particles of various sizes, ranging from micron-sized to those visible to the naked eye. During the emulsification process, system homogenization occurs; however, the resulting emulsion contains not only oil droplets but also aquafaba particles entrapped within the spatial structure of the emulsion. It is not possible to distinguish between aquafaba particles and emulsion droplets solely based on diffraction methods. Therefore, throughout this text, the term “particles” in reference to the emulsion will encompass both oil droplets and aquafaba particles.
A Visco QC 300R rotational viscometer (Anton Paar, Graz, Austria) was used to measure the apparent viscosity following the procedure outlined in [5]. The viscometer is equipped with a concentric cylinder CC12 geometry and a thermostat (Peltier PTD 175, Anton Paar, Graz, Austria) to maintain a controlled temperature of 20 °C. Steady shear viscosity was evaluated within a shear rate range of 0.1–100.0 s−1 for a duration of 120 s. Each measurement was repeated three times.
The emulsifying capacity was determined by the phase inversion point, which corresponds to the maximum amount of fat emulsified in the tested solution before phase inversion occurs. A 100 cm3 beaker was filled with 10 cm3 of aquafaba sample, and then fat was added through a separatory funnel at a rate of 78–80 drops per minute until phase inversion occurred. The volume of fat (cm3) poured from the funnel corresponded to the phase inversion point. The emulsification was performed using an IKA ULTRA-TURRAX T 25 digital homogenizer (3000 rpm). The type of emulsion was determined by the dilution method.
The stability of the aquafaba-based emulsions was determined in two stages. In the first stage, the sample was centrifuged at a rotor speed of 25 s−1 for 5‧60 s. In the second stage, the sample was placed in a water bath at a temperature of 80–85 °C, held for 3‧60 s, and then cooled and centrifuged again for 5‧60 s. The stability of the emulsion was assessed by recording the volumes of phases that separated after centrifugation [12].
The experimental data presented in Table 2 were approximated using logarithmic dependencies. However, the results shown in Figure 5 are presented without the logarithmic relationship to better visualize the pseudoplastic behavior and the differences in viscosity of the samples, depending on their composition. This approach was chosen to avoid redundancy, prevent information overload, and enhance the clarity of data presentation.
2.4. Calculation and Statistical Analysis
All experiments were conducted in triplicate, with results reported as the mean value along with the standard deviation. To determine significant differences between groups, a one-way ANOVA was applied, followed by Tukey’s post hoc test for multiple comparisons, considering a significance level of p < 0.05. Statistical analysis was carried out using SigmaPlot version 15 (Grafiti LLC, Palo Alto, CA, USA).
3. Results and Discussion
3.1. Beans Aquafaba Charateristics
Interest in the commercial production of aquafaba continues to grow. However, additional research is necessary to explore the factors affecting its functional properties and to establish methods for standardizing its large-scale production [13]. Aquafaba consists of dry matter (2.0–5.5%), which includes carbohydrates, particularly starches (mainly amylose), low-molecular-weight proteins, and saponins [13,14].
In a previous study [5], the emulsifying properties of aquafaba derived from canned beans were utilized in the development of low-fat mayonnaise. It was established that the samples exhibited rheological and microstructural properties comparable to those of a commercial mayonnaise product. These experimental results led to investigating the emulsifying properties of freshly cooked aquafaba that was obtained during the technological process of producing the semi-finished product boiled bean grains. This approach allows for an assessment of the functional and technological properties of fresh cooked aquafaba, but the technology may vary significantly between manufacturers in terms of processing parameters and the inclusion of specific raw ingredients. This variation was confirmed in [15], where an analysis of aquafaba from canned chickpeas demonstrated that its chemical composition and functional–technological properties differ significantly depending on the manufacturer. A key drawback of aquafaba obtained from canned legumes is its limited scalability for commercialization on an industrial scale.
At the initial stage of experimental research, it was determined that freshly cooked aquafaba had a dry matter content of 2.8 ± 0.1%, which was insufficient for the formation of emulsions. To increase the dry matter content and the concentration of water-soluble protein, which act as plant-based emulsifiers, a concentration process was applied through simmering at a constant temperature, ensuring that no chemical changes or Maillard reactions occurred. The ability to concentrate dry matter in beans aquafaba shifts the focus away from chickpea-based aquafaba, promoting the use of other legumes as viable sources for its production.
The pH of beans aquafaba was 6.15 ± 0.05, which may indicate the presence of water-soluble organic acids inherent to the raw material. Studies [13,16,17] report similar results, with the aquafaba exhibiting a slightly acidic pH.
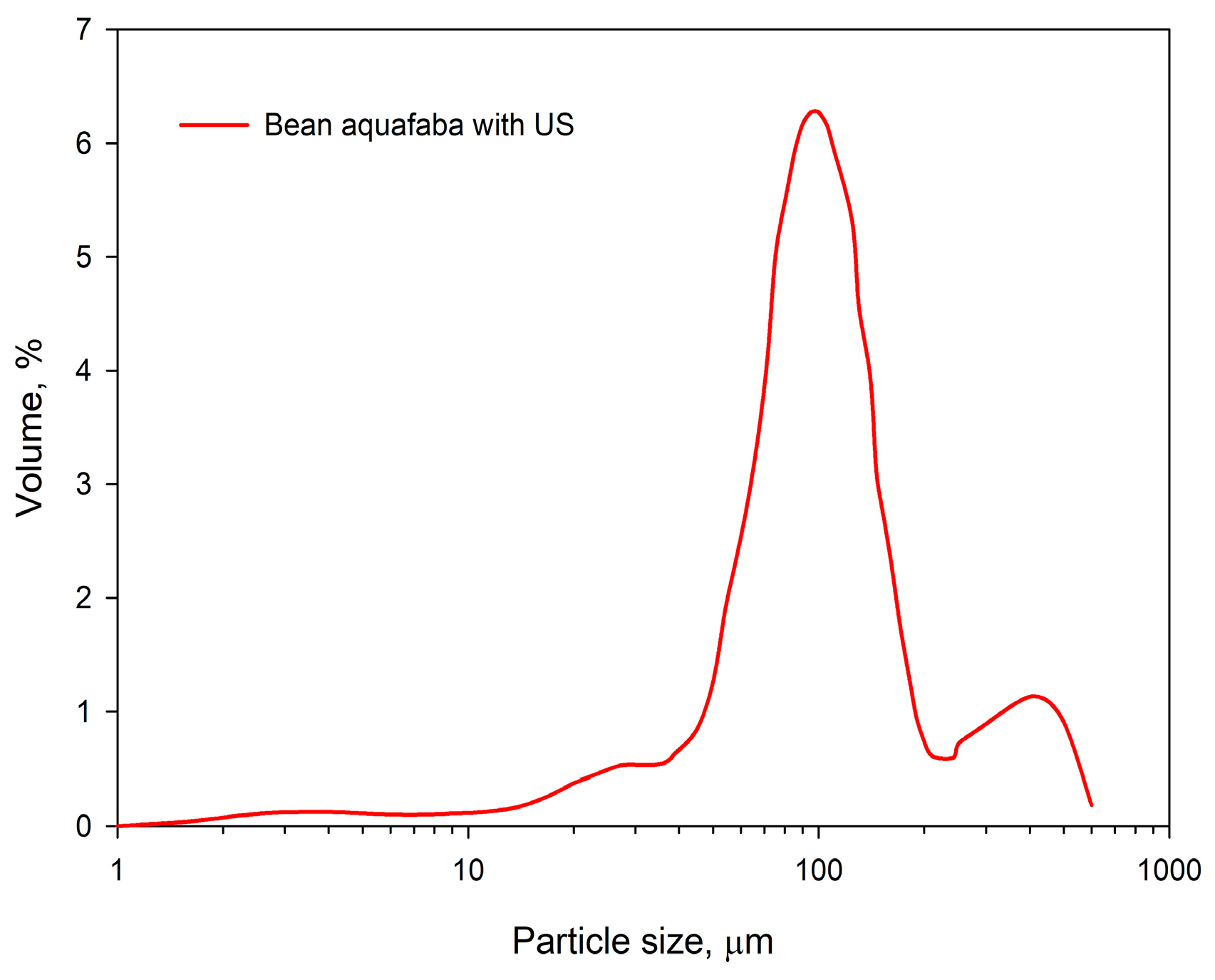
The specified technological parameters allowed for the production of beans aquafaba. Considering its organoleptic properties, it is characterized as a partially transparent liquid without sediment and with a light-yellow color. The aroma is mild, characteristic of boiled legumes, while the taste is neutral with a slight sweet aftertaste. The particle volume-weighted size distribution of beans aquafaba is presented in Figure 2.
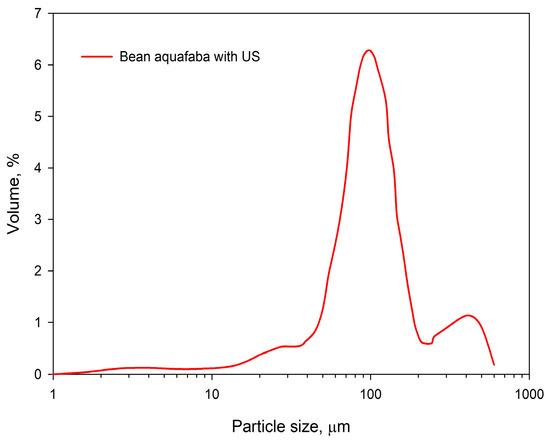
Figure 2.
Volume-weighted size distribution of beans aquafaba was treated with ultrasound.
The particle size distribution curve of the analyzed beans aquafaba displayed a peak between 90.8 ± 0.4 µm and 104.3 ± 0.4 µm, with a median diameter 77.9 ± 0.4 µm and a distribution span of 1.47. The obtained volume-weighted particle size distribution of beans aquafaba may reflect the biological characteristics of the selected legumes and potential microdamage to the cotyledon caused by hydrothermal processing.
Beans aquafaba demonstrates a high emulsifying capacity, which depends on the mass fraction of dry solids in its composition. For aquafaba with a mass fraction of dry solids of 2.8 ± 0.1%, the emulsifying capacity is 70 ± 1%. Increasing the mass fraction of dry solids to 4.2 ± 0.1% correlates with an increase in emulsifying capacity to 84 ± 1%. However, further increases in the mass fraction of dry solids in aquafaba did not lead to significant changes in the emulsifying capacity, indicating the achievement of a peak effect of the studied parameters on each other.
Considering the obtained data on emulsifying capacity, our further research focused on emulsions with 30% and 60% fat content. The choice of this range is driven by the need to obtain a broader spectrum of experimental data on the impact of the fat mass fraction on the structural–mechanical and rheological properties, which is crucial for practical application in the food industry.
3.2. Characteristics of Emulsions Based on Beans Aquafaba
The volume-weighted size distribution curve of the emulsion with a fat content of 30% has two peaks, one of which is in the range of 2.3 ± 0.1 µm to 3.1 ± 0.1 µm, and the second peak is in the range of 19.1 ± 0.3 µm to 20.2 ± 0.4 µm, with percentile values of D10 = 1.4 ± 0.01 µm, D50 = 5.3 ± 0.1 µm, and D90 = 15.7 ± 0.3 µm. In contrast, emulsions with a 60% fat content had a wider distribution with percentile values of D10 = 1.7 ± 0.1 µm, D50 = 8.5 ± 0.2 µm, and D90 = 18.1 ± 0.3 µm. The observed increase in the median particle size of the emulsion may be attributed to the insufficient emulsifying content in the aquafaba, which plays a key role in stabilizing the emulsion.
This can lead to the formation of larger particles. Although the existing protein content in beans aquafaba is sufficient to form emulsions, phase separation was observed over time. This could be attributed to the insufficient viscosity of the continuous phase and the lack of sufficient amounts of stabilizing components, as the protein content directly in the emulsions was only 0.3–0.6%. At the same time, particles coated with a protein–polysaccharide mixed layer generally exhibit greater stability compared to those coated only with proteins, separate polysaccharide layers or layer-by-layer structures. This can be explained by changes in charge, structure, and an increase in the thickness of the interfacial layer, which can affect the stability of the emulsions over time [18]. The above highlights the necessity of adding stabilizers to ensure the long-term stability of emulsions based on beans aquafaba.
3.3. The Stability of Emulsion Systems Based on Chickpea Aquafaba
For the stabilization of emulsion systems, xanthan gum and pregelatinized corn starch were chosen as stabilizers. According to the theory of emulsion stability, these stabilizers enable the achievement of physical stability, and from a technological perspective, they ensure the production of emulsions with excellent structural–mechanical properties. This is crucial for achieving the desired organoleptic properties of the final product, in which the emulsion system will be incorporated.
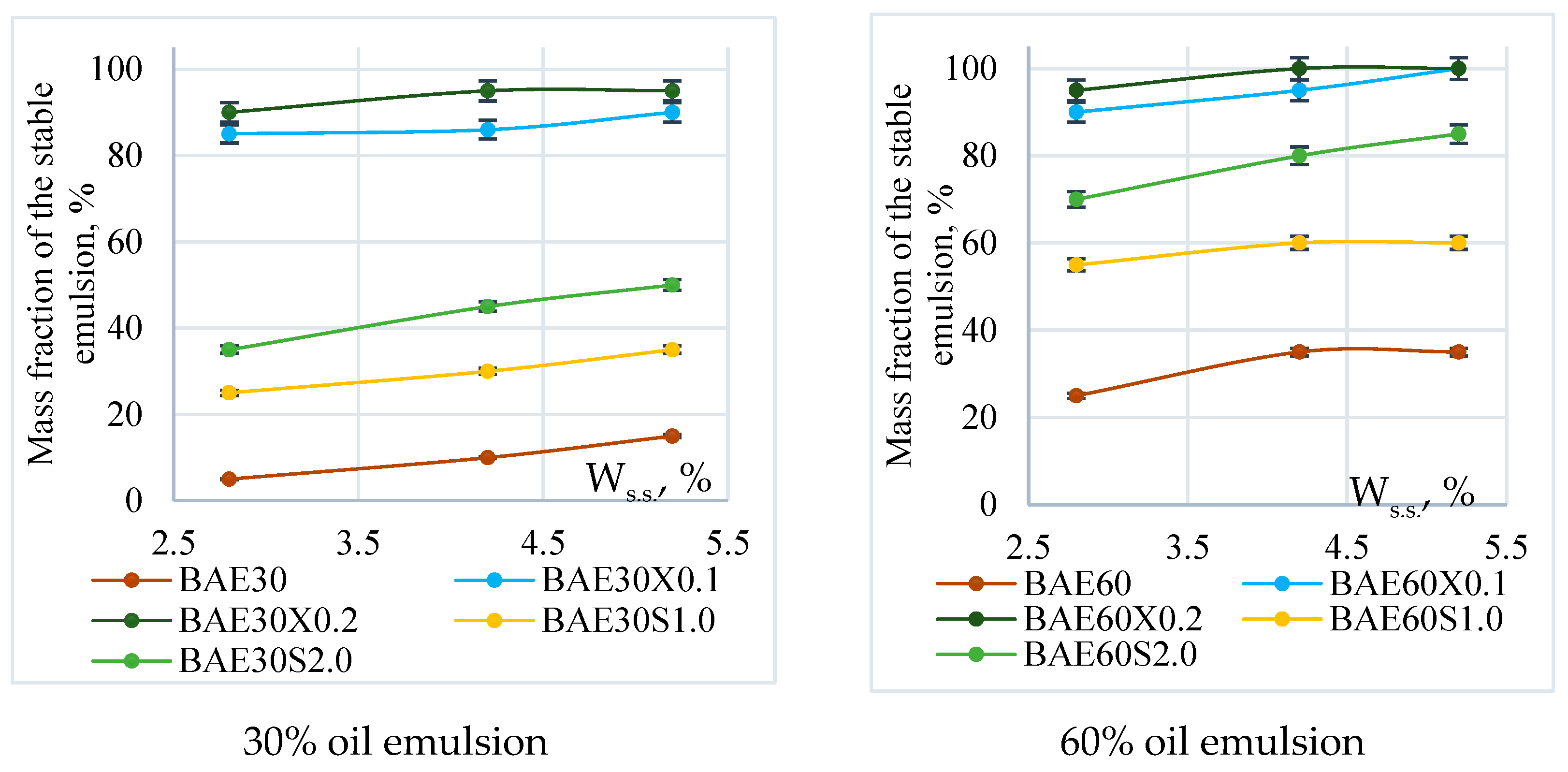
Figure 3 illustrates the effect of the mass fraction of dry solids in aquafaba on the stability of emulsion systems with 30% and 60% fat content, both with and without the chosen stabilizers.
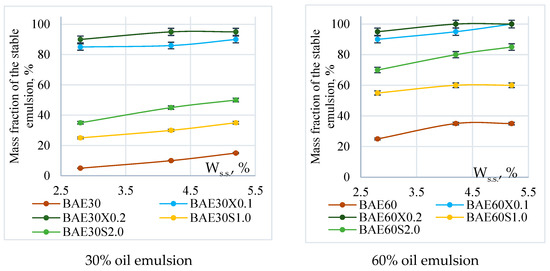
Figure 3.
Emulsion stability.
Stable emulsions were not obtained without the use of stabilizers. In contrast, 100% emulsion stability was achieved by adding 0.2% xanthan gum, regardless of the mass fraction of the fat phase or the dry matter content in the aquafaba. Using pregelatinized corn starch, the maximum stability of the system (80–85%) was achieved only in emulsions with 60% fat content, where aquafaba with a dry matter content of 4.2 ± 0.1% and 5.2 ± 0.2% was used.
The studied systems were characterized by a pH value of 5.9 ± 0.1.
Considering the experimental data on emulsifying capacity and the stability of emulsions, our further research focused on aquafaba with a dry matter content of 4.2 ± 0.1%, which corresponds to a protein content of 0.8% in aquafaba, or 19.04% when expressed on a dry matter basis.
3.4. Characteristics of the Impact of Stabilizers on Emulsion Size Properties
The particle size of emulsions incorporated into food products significantly affects the overall shelf life, sensory properties and textural characteristics of the final product [19].
The Kalliope software for the PSA 1190 device calculates the particle size distribution based on laser diffraction measurements, assuming that the scattered particles are spherical and homogeneous [19]. Accordingly, considering the number of samples analyzed, the results obtained using this equipment accurately represent the particle size distribution. However, to identify specific destabilization processes, the application of additional methods is required.
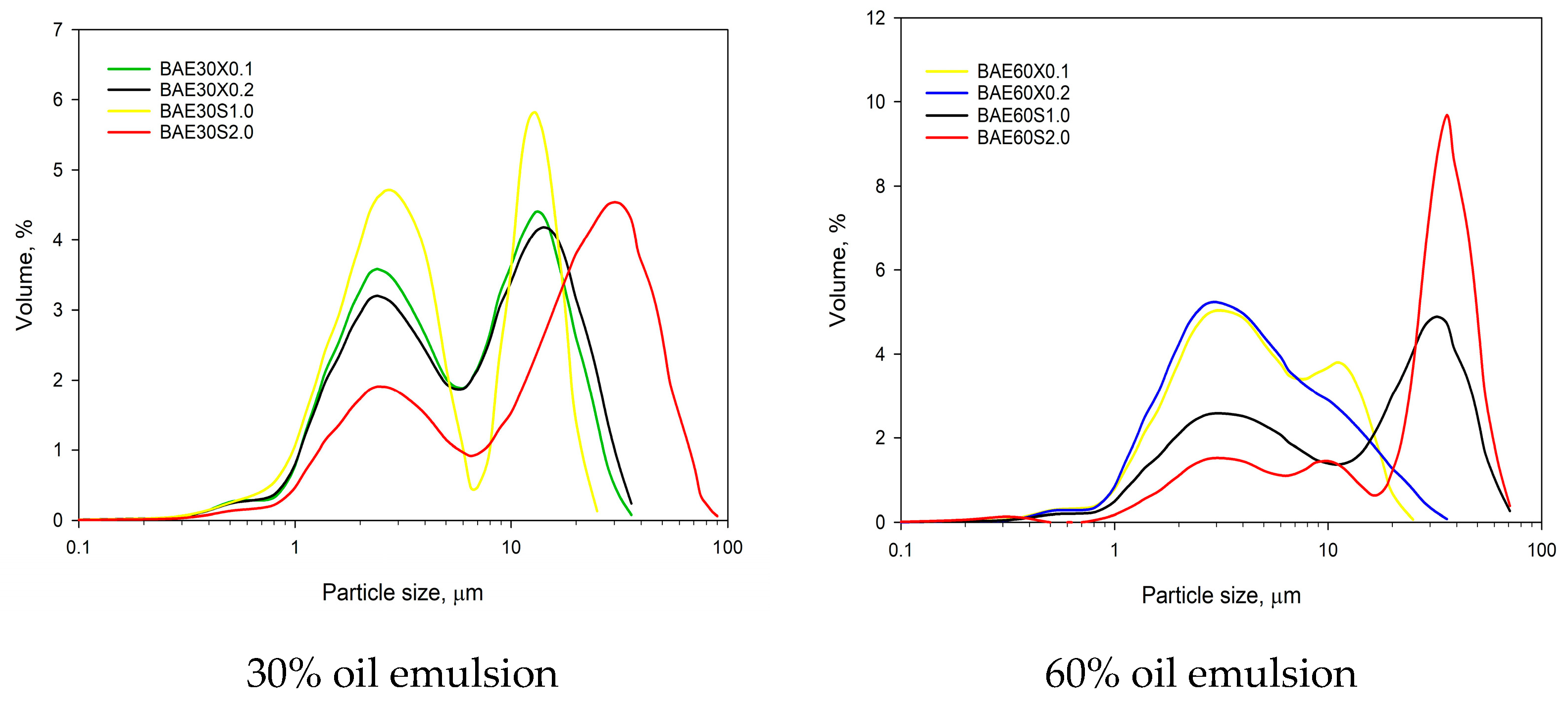
Samples of emulsions with a fat content of 30% and xanthan gum concentrations (0.1–0.2%) as a stabilizer exhibited a bimodal particle size distribution, with peaks at approximately 2.8 ± 0.1 µm and 10.5 ± 0.1 µm. The introduction of 2% pregelatinized corn starch as a stabilizer resulted in a pronounced peak at around 27.9–32.9 µm. Increasing the fat content to 60%, combined with xanthan gum stabilization, led to a leftward shift of the curve, with a peak appearing at approximately 2.6–3.2 µm, indicating a reduction in the median particle size of the emulsion. Conversely, when pregelatinized corn starch was used to stabilize the 60% fat emulsion, an increase in the volume-weighted particle size distribution was observed, with a peak appearing at approximately 35.2–36.7 µm.
The addition of 0.1% xanthan gum to emulsions (BAE30X0.1) with a fat content of 30% resulted in samples with a volume-weighted median diameter of 4.7 ± 0.1 µm (Figure 4), which is equivalent to the samples without the addition of polysaccharide. However, increasing the polysaccharide content to 0.2% raised the median diameter to 5.4 ± 0.1 µm (BAE30X0.2). Increasing the fat content while using xanthan gum had a minimal effect on the median diameter, which remained in the range of 3.7–3.8 µm. Xanthan gum facilitates the adsorption of proteins present in aquafaba, enhances the viscosity of the continuous phase and reduces the intensity of Brownian motion, thereby contributing to improved emulsion stability.
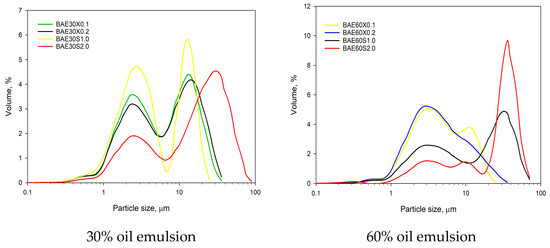
Figure 4.
Volume-weighted particle size distribution of beans aquafaba emulsions.
The addition of 1% (BAE30S1.0) and 2% (BAE30S2.0) pregelatinized corn starch to emulsions with a fat content of 30% resulted in median diameters of 3.3 ± 0.1 µm and 15.8 ± 0.4 µm, respectively. In emulsions with a fat content of 60%, the median diameters were 11.6 ± 0.3 µm and 28.6 ± 0.2 µm. This effect of pregelatinized starch can be explained by the swelling of starch granules in the aqueous phase of the emulsion system, which in turn, affects the observed characteristics.
The rheological properties of emulsions, particularly their viscosity, are profoundly influenced by the characteristics of their dispersed phase. Specifically, the particle size distribution of droplets within an emulsion plays a critical role in dictating its flow behavior. Therefore, comprehensive viscometry studies must be complemented by precise particle sizing techniques to establish robust structure–property relationships, allowing for a deeper understanding and targeted optimization of emulsion stability.
3.5. The Viscosity Characteristics of Emulsions
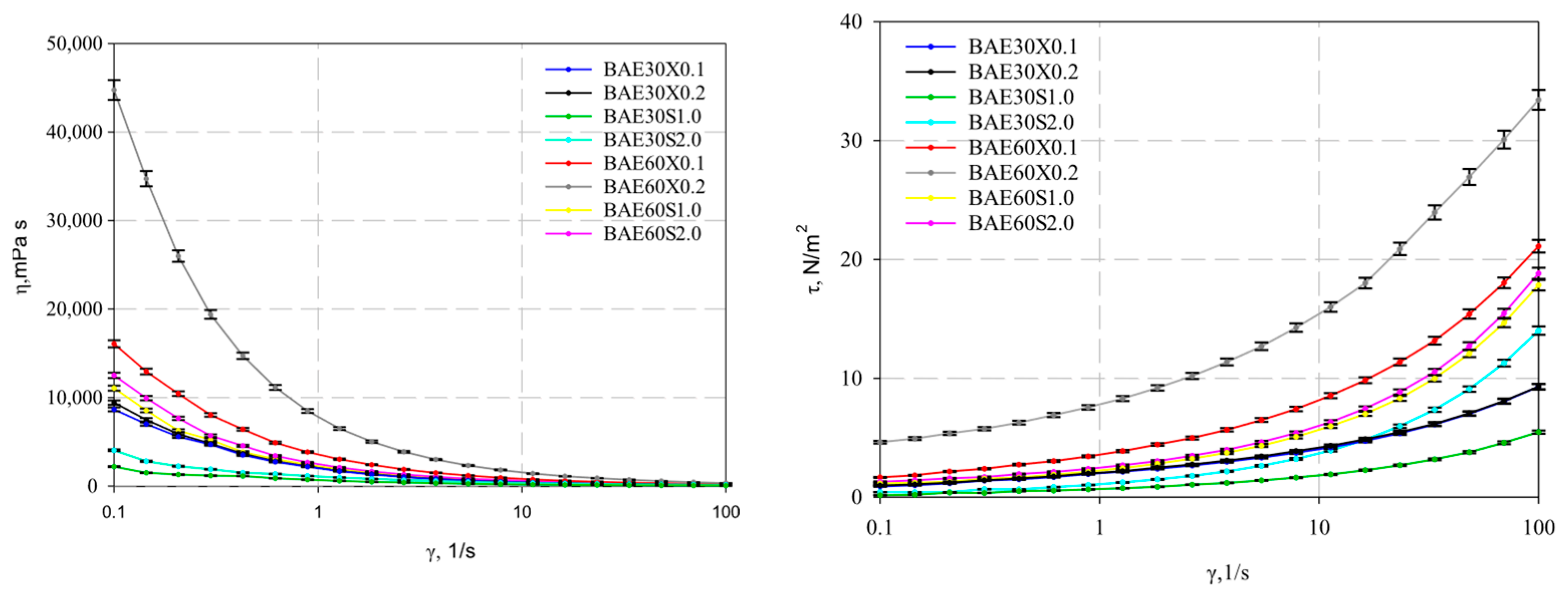
The experimental data of the determined rheological properties of the emulsion systems are presented in Figure 5.
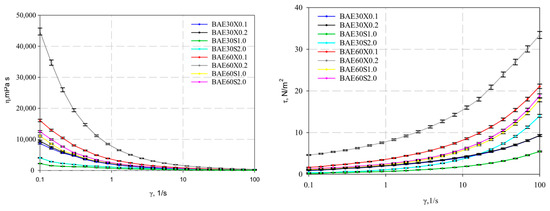
Figure 5.
Viscosity and shear stress as a function of shear rate.
The presented figure illustrates that all studied systems exhibit shear-thinning (pseudoplastic) behavior, as evidenced by the decrease in viscosity with increasing shear rate.
It was determined that, at a shear rate of 16.24 s−1, the systems reach a critically disrupted structure (Figure 5). Specifically, the emulsion system with 60% fat content stabilized with 0.2% xanthan gum exhibits the highest viscosity, which is 713.5 ± 19.5 mPa·s at a shear rate of 33.6 s−1. In contrast, the lowest viscosity was observed in the emulsion system with 30% fat content stabilized with 1% pregelatinized corn starch, which is 94.8 ± 2.8 mPa·s at a shear rate of 33.6 s−1.
The flow curves obtained for the investigated emulsions were approximated using the power-law and Herschel–Bulkley equations. The analysis of experimental data with the selected rheological models showed high correlation coefficients (R2), indicating their strong fitting accuracy and high approximation reliability.
The calculated dynamic yield stress consistently increased with the rising content of both oil and thickener, ranging from 0.3 to 5.0 Pa.
The studied emulsion samples exhibited pseudoplastic flow behavior, as confirmed by the values presented in Table 2, where the flow behavior index for all samples was less than one. The highest consistency index values, coupled with the smallest particle size distribution weighted by the volume D[4,3], were achieved in samples stabilized with xanthan gum. This indicates it was the strongest and most time-stable structure among the tested formulations. However, it can generally be stated that the data in Table 2 regarding emulsions with 30% fat content and stabilization with 0.1–0.2% xanthan gum lie within the error margin, which may indicate the feasibility of increasing the stabilizer content until the critical concentration is reached, leading to a significant increase in viscosity.

Table 2.
Microstructural and rheological characteristics of emulsion samples.
It is worth noting that in emulsions with a 30% oil content, the addition of xanthan gum had a significant impact on viscosity, while in emulsions with a 60% oil content, the addition of pregelatinized corn starch played a key role. The determined consistency coefficients reflect these patterns. Thus, higher concentrations of the selected polysaccharides resulted in more viscous systems with improved stability. Considering that the polysaccharides used introduce variability in textural and viscosity characteristics, the resulting emulsions can be applied in the technology of a wide range of food products.
4. Conclusions
Aquafaba, due to its content of water-soluble proteins and other nutrients, has evolved from a by-product of legume processing into an innovative food ingredient.
Studies have shown that beans aquafaba can be used to form stable food emulsions. Specifically, aquafaba with a dry matter content of 2.8 ± 0.1% has an emulsion capacity of 70 ± 1%, while when the dry matter content in aquafaba increases to 4.2 ± 0.1%, the emulsion capacity rises to 84 ± 1%.
It was determined that emulsions achieved 95–100% stability, regardless of the fat content, with the addition of 0.2% xanthan gum. In contrast, using pregelatinized corn starch, 80–85% stability was achieved only in emulsions with 60% fat content.
The influence of oil content and different stabilizers on microstructure and rheological characteristics of food emulsions based on beans aquafaba was investigated. It was determined that emulsions with 30% fat content, stabilized with 0.1–0.2% xanthan gum, have a volume-weighted median diameter of 4.7 ± 0.1 µm and 5.4 ± 0.1 µm, with a viscosity of 182.3–183.6 mPa·s at a shear rate of 33.6 s−1. Increasing the fat content to 60% allowed the production of emulsions with a volume-weighted median diameter of 3.7–3.8 µm, and an increase in viscosity to 392.5 ± 11.4 mPa·s and 713.5 ± 19.5 mPa·s at a shear rate of 33.6 s−1. Thus, increasing the xanthan gum content did not have a significant effect on the microstructural properties of emulsions with 60% fat content. The experimental data indicate the high effectiveness of using xanthan gum in emulsion systems based on beans aquafaba, particularly when the fat content is 60%.
In emulsions with 30% fat content, using 1–2% pregelatinized corn starch, the median diameter ranged from 3.3 ± 0.1 µm to 15.8 ± 0.4 µm, while for emulsions with 60% fat content, the median diameter ranged from 11.6 ± 0.3 µm to 28.6 ± 0.2 µm. The obtained data may indicate the swelling of the pregelatinized corn starch granules in the continuous phase. The viscosity of these systems increased from 94.8 ± 2.8 and 219.2 ± 6.5 mPa·s to 297.7 ± 8.9 and 314.0 ± 9.4 mPa·s, respectively, at a shear rate of 33.6 s−1, due to the increase in fat content from 30% to 60%.
This research expands the existing knowledge about the functional and technological properties of aquafaba from various legumes, paving the way for its commercialization in the food industry.
Author Contributions
Conceptualization, O.H. and A.R.; methodology, S.G. and O.H.; software, S.G. and V.D.; validation, S.G., O.H. and A.S.; formal analysis, S.G. and V.D.; investigation, A.S., A.R. and V.D.; data curation, O.H.; writing—original draft preparation, A.R. and V.D.; writing—review and editing, S.G., A.S., A.R. and O.H.; visualization, V.D.; supervision, A.R.; project administration, O.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Simons Foundation, grant award number 1290597.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Data presented in this study are available on request from the corresponding author.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to thank Volodymyr Pashko (DonauLab Ukraine, Kyiv, Ukraine) for his support in the experimental procedures.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Radchenko, A.; Dehtiar, V. Technological aspects of the use of legumes in the technology of food products with an emulsion structure. In Responsible Production and Consumption: Realization in New Generations of Food Products: Scientific Monograph; Baltija Publishing: Riga, Latvia, 2024; pp. 90–158. [Google Scholar] [CrossRef]
- Bochenek, H.; Francis, N.; Santhakumar, A.; Blanchard, C.; Chinkwo, K. The antioxidant and anticancer properties of chickpea water and chickpea polyphenol extracts in vitro. Cereal Chem. 2023, 100, 895–903. [Google Scholar] [CrossRef]
- Huang, Y.-P.; Masarweh, C.; Paviani, B.; Mills, D.; Barile, D. Exploring bioactive compounds in chickpea and bean aquafaba: Insights from glycomics and peptidomics analyses. Food Chem. 2024, 460, 140635. [Google Scholar] [CrossRef] [PubMed]
- Alfaro-Diaz, A.; Escobedo, A.; Luna-Vital, D.; Castillo-Herrera, G.; Mojica, L. Common beans as a source of food ingredients: Techno-functional and biological potential. Compr. Rev. Food Sci. Food Saf. 2023, 22, 2910–2944. [Google Scholar] [CrossRef] [PubMed]
- Sachko, A.; Sema, O.; Grinchenko, O.; Gubsky, S. Canned Beans Aquafaba as an Egg White Substitute in the Technology of Low-Fat Mayonnaise. Eng. Proc. 2023, 56, 206. [Google Scholar] [CrossRef]
- Grossi Bovi Karatay, G.; Medeiros Theóphilo Galvão, A.M.; Dupas Hubinger, M. Storage Stability of Conventional and High Internal Phase Emulsions Stabilized Solely by Chickpea Aquafaba. Foods 2022, 11, 1588. [Google Scholar] [CrossRef] [PubMed]
- Grossi Bovi Karatay, G.; Rebellato, A.P.; Joy Steel, C.; Dupas Hubinger, M. Chickpea Aquafaba-Based Emulsions as a Fat Replacer in Pound Cake: Impact on Cake Properties and Sensory Analysis. Foods 2022, 11, 2484. [Google Scholar] [CrossRef] [PubMed]
- Plant-Based Food Market Outlook (2023 to 2033). Available online: https://surl.li/iwdznx (accessed on 5 January 2025).
- Hrynchenko, O.; Dehtiar, V.; Radchenko, A.; Pak, A.; Smetanska, I.; Percevoy, F. Revealing the Effect of Hydrothermal Processing of Legumes on the Accumulation of Dry Matter in Aquafaba. East.-Eur. J. Enterp. Technol. 2024, 5, 51–61. [Google Scholar] [CrossRef]
- Gornall, A.G.; Bardawill, C.J.; David, M.M. Determination of serum proteins by means of the biuret reaction. J. Biol. Chem. 1949, 177, 751–766. [Google Scholar] [CrossRef] [PubMed]
- ISO 13320:2020; Particle Size Analysis—Laser Diffraction Methods. International Standard Confirmed [90.93]. Edition 2; International Organization for Standardization (ISO): Geneva, Switzerland, 2020; pp. 1–59. Available online: https://www.iso.org/standard/69111.html (accessed on 1 January 2025).
- DSTU 4560:2006; Mayonnaises. Acceptance Rules and Testing Methods. Effective from 2008-01-01. Official Edition; UkrNDNC: Kyiv, Ukraine, 2008; pp. 1–16. Available online: https://online.budstandart.com/ua/catalog/doc-page?id_doc=92570 (accessed on 5 January 2025).
- He, Y.; Meda, V.; Reaney, M.J.T.; Mustafa, R. Aquafaba, a New Plant-Based Rheological Additive for Food Applications. Trends Food Sci. Technol. 2021, 111, 27–42. [Google Scholar] [CrossRef]
- Ramos-Figueroa, J.; Tse, T.; Shen, J.; Purdy, S.; Kim, J.; Kim, Y.; Han, B.; Hong, J.; Shim, Y.; Reaney, M. Foaming with Starch: Exploring Faba Bean Aquafaba as a Green Alternative. Foods 2023, 12, 3391. [Google Scholar] [CrossRef] [PubMed]
- Shim, Y.; Mustafa, R.; Shen, J.; Ratanapariyanuch, K.; Reaney, M. Composition and Properties of Aquafaba: Water Recovered from Commercially Canned Chickpeas. J. Vis. Exp. 2018, 132, 56305. [Google Scholar] [CrossRef]
- He, Y.; Shim, Y.Y.; Shen, J.; Kim, J.H.; Cho, J.Y.; Hong, W.S.; Meda, V.; Reaney, M.J.T. Aquafaba from Korean Soybean II: Physicochemical Properties and Composition Characterized by NMR Analysis. Foods 2021, 10, 2589. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.M.N.; Ngọc, N.P.; Quoc, L.P.T.; Tran, G.B. Application of Chickpeas Aquafaba with Pre-treatment as Egg Replacer in Cake Production. Chem. Eng. Trans. 2021, 89, 7–12. [Google Scholar] [CrossRef]
- Guzey, D.; McClements, D.J. Impact of electrostatic interactions on formation and stability of emulsions containing oil droplets coated by beta-lactoglobulin-pectin complexes. J. Agric. Food Chem. 2007, 55, 475–485. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Food Emulsions, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2015; ISBN 9780429154034. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).