Effect of Weight Distribution on Knee Joint Temperature Pattern Under Fatigue Condition †
Abstract
1. Introduction
2. Materials and Methods
2.1. Equipment
2.2. Participants
2.3. Procedure
3. Results and Discussion
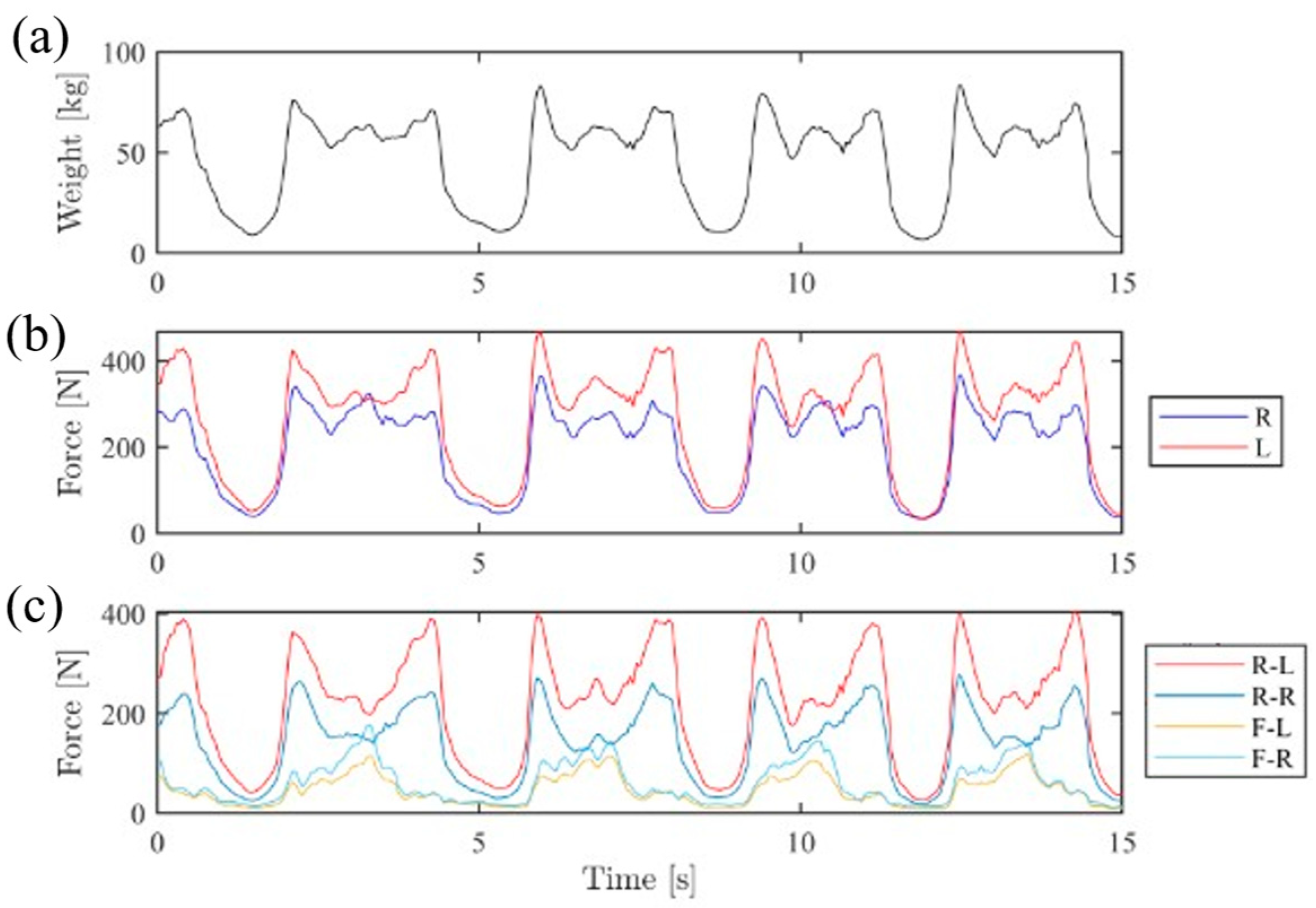
3.1. Weight Distribution
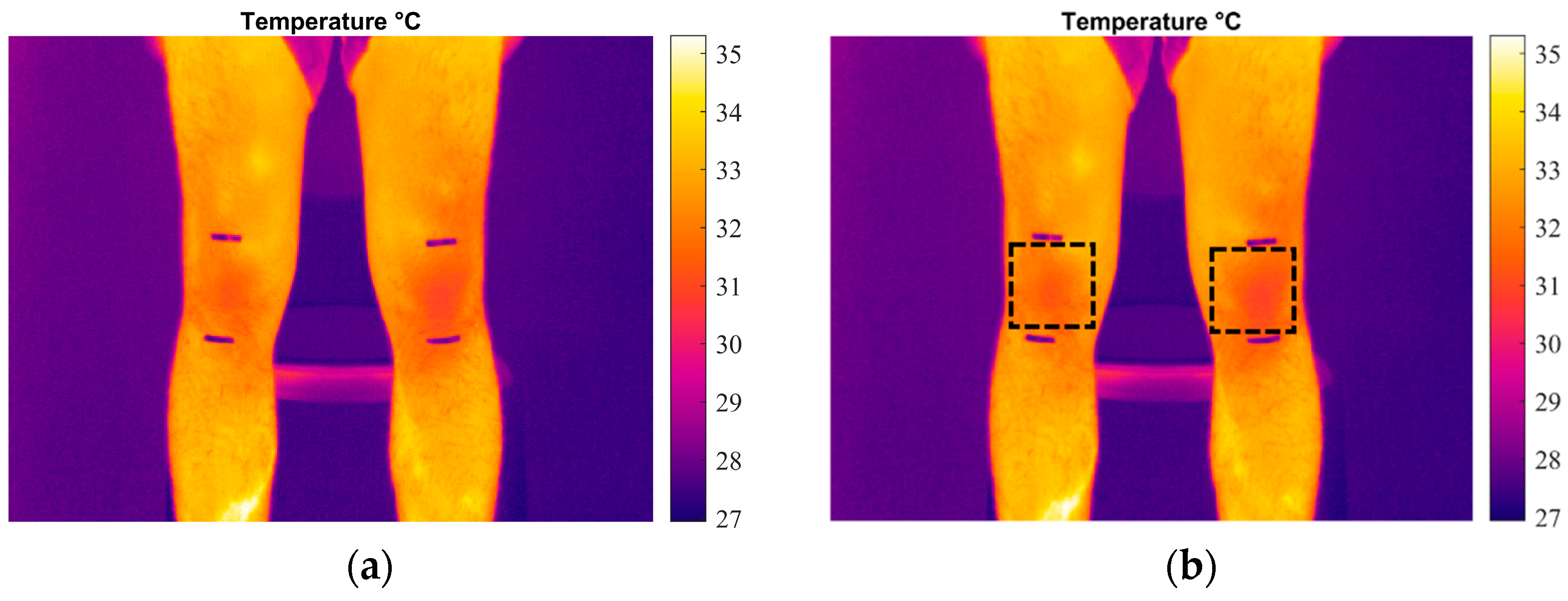
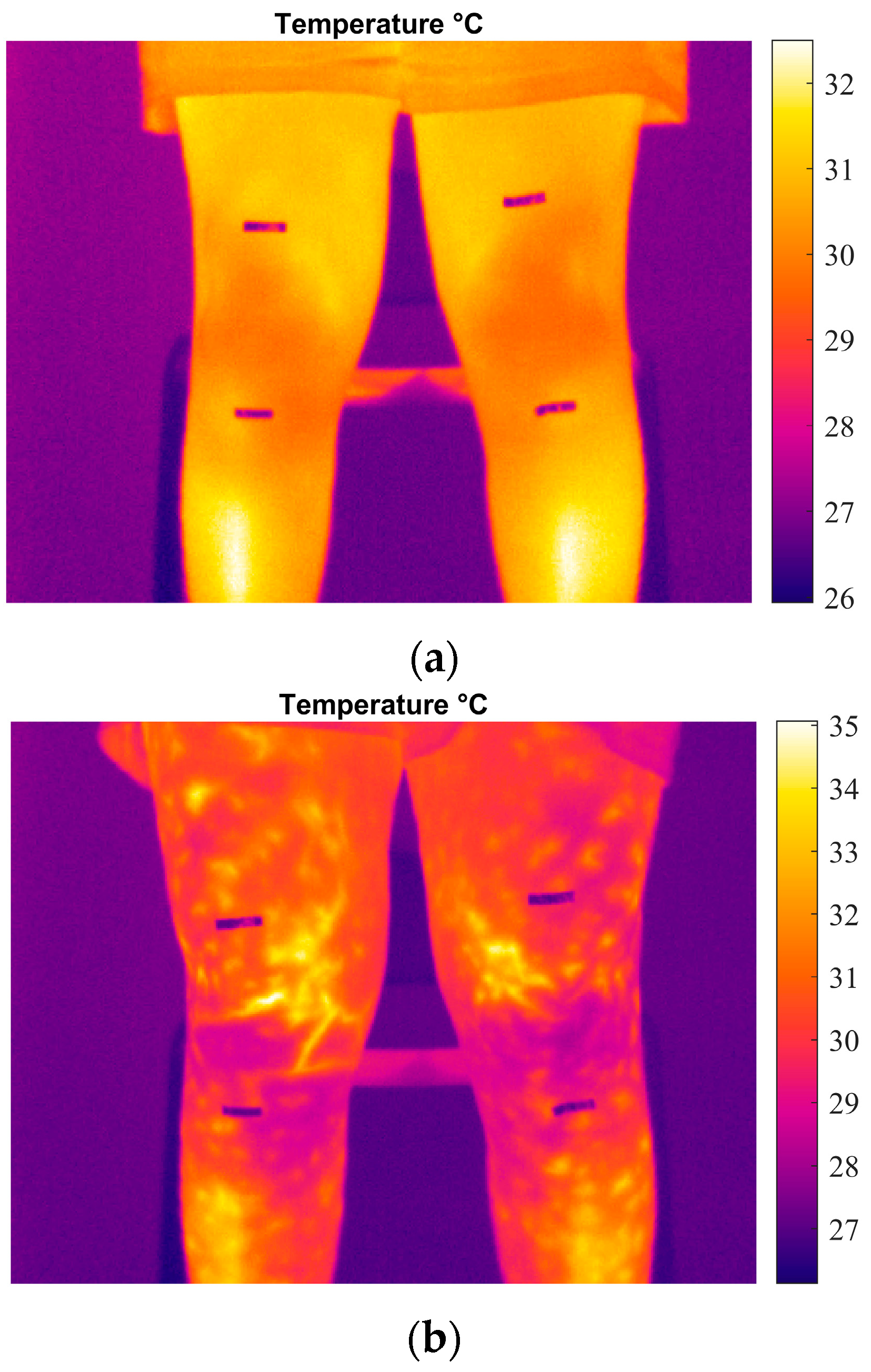
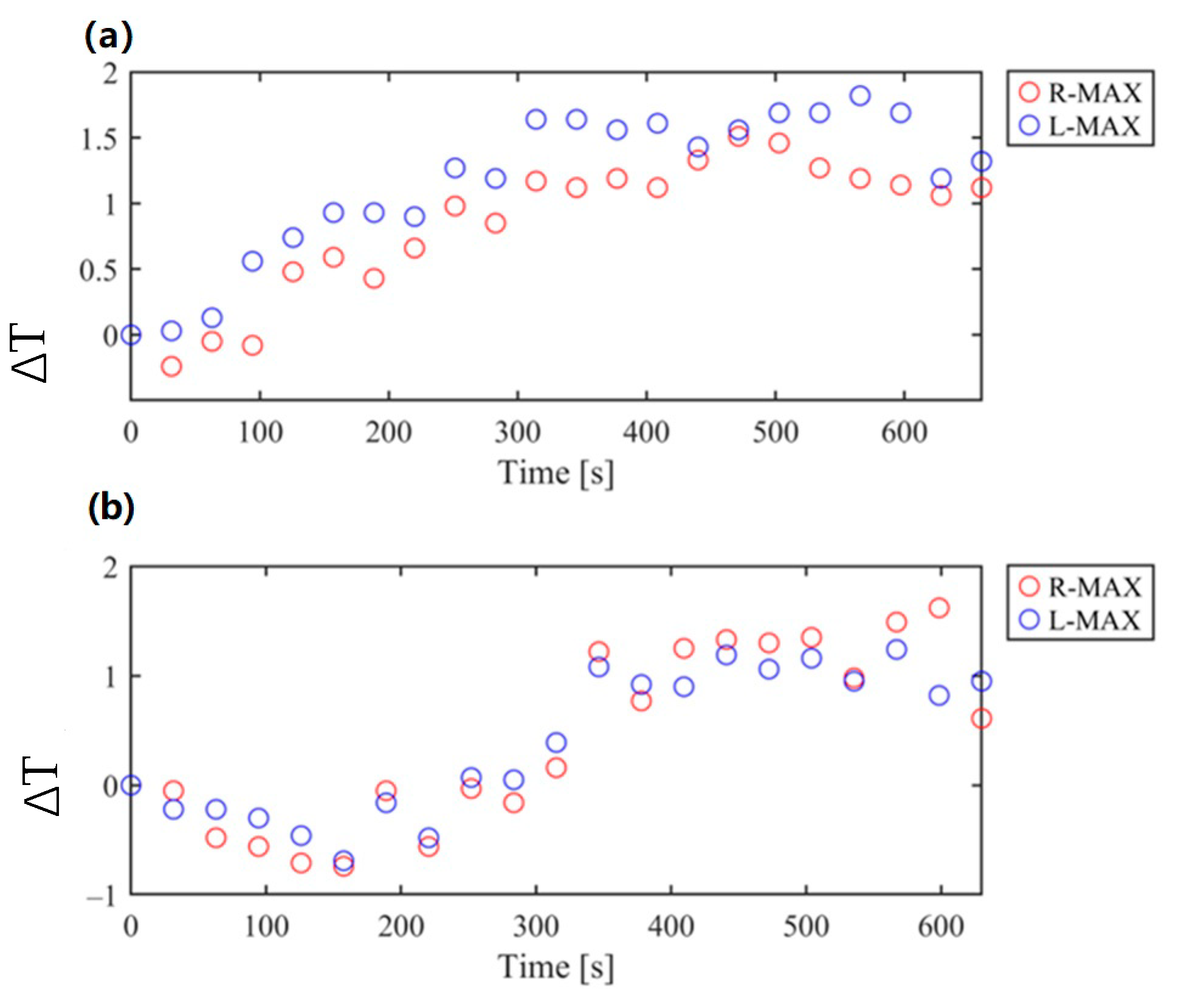
3.2. Temperature Monitoring
4. Observations and Future Works
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Denoble, A.E.; Hall, N.; Pieper, C.F.; Kraus, V.B. Patellar Skin Surface Temperature by Thermography Reflects Knee Osteoarthritis Severity. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2010, 3, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Geneva, I.I.; Cuzzo, B.; Fazili, T.; Javaid, W. Normal Body Temperature: A Systematic Review. Open Forum Infect. Dis. 2019, 6, ofz032. [Google Scholar] [CrossRef]
- De Marziani, L.; Boffa, A.; Angelelli, L.; Andriolo, L.; Di Martino, A.; Zaffagnini, S.; Filardo, G. Infrared Thermography in Symptomatic Knee Osteoarthritis: Joint Temperature Differs Based on Patient and Pain Characteristics. J. Clin. Med. 2023, 12, 2319. [Google Scholar] [CrossRef]
- Crisafulli, D.; Spataro, M.; De Marchis, C.; Risitano, G.; Milone, D. A New Sensorized Approach Based on a DeepLabCut Model and IR Thermography for Characterizing the Thermal Profile in Knees During Exercise. Sensors 2024, 24, 7862. [Google Scholar] [CrossRef]
- Hildebrandt, C.; Zeilberger, K.; Ring, E.F.J.; Raschner, C.; Hildebrandt, C.; Zeilberger, K.; Ring, E.F.J.; Raschner, C. The Application of Medical Infrared Thermography in Sports Medicine. Int. Perspect. Top. Sports Med. Sports Inj. 2012. [Google Scholar] [CrossRef]
- Calin, M.A.; Mologhianu, G.; Savastru, R.; Calin, M.R.; Brailescu, C.M. A Review of the Effectiveness of Thermal Infrared Imaging in the Diagnosis and Monitoring of Knee Diseases. Infrared Phys. Technol. 2015, 69, 19–25. [Google Scholar] [CrossRef]
- Hildebrandt, C.; Raschner, C.; Ammer, K. An Overview of Recent Application of Medical Infrared Thermography in Sports Medicine in Austria. Sensors 2010, 10, 4700–4715. [Google Scholar] [CrossRef] [PubMed]
- Milone, D.; D’Andrea, D.; Santonocito, D. Smart Design of Hip Replacement Prostheses Using Additive Manufacturing and Machine Learning Techniques. Prosthesis 2024, 6, 24–40. [Google Scholar] [CrossRef]
- Milone, D.; Risitano, G.; Pistone, A.; Crisafulli, D.; Alberti, F. A New Approach for the Tribological and Mechanical Characterization of a Hip Prosthesis Trough a Numerical Model Based on Artificial Intelligence Algorithms and Humanoid Multibody Model. Lubricants 2022, 10, 160. [Google Scholar] [CrossRef]
- Mangine, R.E.; Siqueland, K.A.; Noyes, F.R. The Use of Thermography for the Diagnosis and Management of Patellar Tendinitis. J. Orthop. Sports Phys. Ther. 1987, 9, 132–140. [Google Scholar] [CrossRef][Green Version]
- Molina-Payá, F.J.; Ríos-Díaz, J.; Carrasco-Martínez, F.; Martínez-Payá, J.J. Infrared Thermography, Intratendon Vascular Resistance, and Echotexture in Athletes with Patellar Tendinopathy: A Cross-Sectional Study. Ultrason. Imaging 2023, 45, 47–61. [Google Scholar] [CrossRef]
- Chaudhry, S.; Fernando, R.; Screen, H.; Waugh, C.; Tucker, A.; Morrissey, D. The Use of Medical Infrared Thermography in the Detection of Tendinopathy: A Systematic Review. Phys. Ther. Rev. 2016, 21, 75–82. [Google Scholar] [CrossRef]
- Molina-Payá, J.; Ríos-Díaz, J.; Martínez-Payá, J. Inter and Intraexaminer Reliability of a New Method of Infrared Thermography Analysis of Patellar Tendon. Quant. Infrared Thermogr. J. 2021, 18, 127–139. [Google Scholar] [CrossRef]
- Arfaoui, A.; Bouzid, M.A.; Pron, H.; Taiar, R.; Polidori, G. Application of Infrared Thermography as a Diagnostic Tool of Knee Osteoarthritis. J. Therm. Sci. Technol. 2012, 7, 227–235. [Google Scholar] [CrossRef][Green Version]
- Schiavon, G.; Capone, G.; Frize, M.; Zaffagnini, S.; Candrian, C.; Filardo, G. Infrared Thermography for the Evaluation of Inflammatory and Degenerative Joint Diseases: A Systematic Review. Cartilage 2021, 13, 1790S–1801S. [Google Scholar] [CrossRef] [PubMed]
- Milone, D.; Longo, F.; Merlino, G.; De Marchis, C.; Risitano, G.; D’Agati, L. MocapMe: DeepLabCut-Enhanced Neural Network for Enhanced Markerless Stability in Sit-to-Stand Motion Capture. Sensors 2024, 24, 3022. [Google Scholar] [CrossRef] [PubMed]
- Menezes, P.; Rhea, M.R.; Herdy, C.; Simão, R. Effects of Strength Training Program and Infrared Thermography in Soccer Athletes Injuries. Sports 2018, 6, 148. [Google Scholar] [CrossRef] [PubMed]
- Trejo-Chavez, O.; Amezquita-Sanchez, J.P.; Huerta-Rosales, J.R.; Morales-Hernandez, L.A.; Cruz-Albarran, I.A.; Valtierra-Rodriguez, M. Automatic Knee Injury Identification through Thermal Image Processing and Convolutional Neural Networks. Electronics 2022, 11, 3987. [Google Scholar] [CrossRef]
- Fernández-Cuevas, I.; Bouzas Marins, J.C.; Arnáiz Lastras, J.; Gómez Carmona, P.M.; Piñonosa Cano, S.; García-Concepción, M.Á.; Sillero-Quintana, M. Classification of Factors Influencing the Use of Infrared Thermography in Humans: A Review. Infrared Phys. Technol. 2015, 71, 28–55. [Google Scholar] [CrossRef]
- Severini, G.; Straudi, S.; Pavarelli, C.; Da Roit, M.; Martinuzzi, C.; Di Marco Pizzongolo, L.; Basaglia, N. Use of Nintendo Wii Balance Board for Posturographic Analysis of Multiple Sclerosis Patients with Minimal Balance Impairment. J. Neuroeng. Rehabil. 2017, 14, 1–14. [Google Scholar] [CrossRef]
- D’andrea, D.; Cucinotta, F.; Farroni, F.; Risitano, G.; Santonocito, D.; Scappaticci, L. Development of Machine Learning Algorithms for the Determination of the Centre of Mass. Symmetry 2021, 13, 401. [Google Scholar] [CrossRef]
- Clark, R.A.; Mentiplay, B.F.; Pua, Y.H.; Bower, K.J. Reliability and Validity of the Wii Balance Board for Assessment of Standing Balance: A Systematic Review. Gait Posture 2018, 61, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Simmons, G.H.; Wong, B.J.; Holowatz, L.A.; Kenney, W.L. Changes in the Control of Skin Blood Flow with Exercise Training: Where Do Cutaneous Vascular Adaptations Fit In? Exp. Physiol. 2011, 96, 822–828. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spataro, M.; Crisafulli, D.; Marchis, C.D.; Risitano, G.; Milone, D. Effect of Weight Distribution on Knee Joint Temperature Pattern Under Fatigue Condition. Eng. Proc. 2025, 85, 43. https://doi.org/10.3390/engproc2025085043
Spataro M, Crisafulli D, Marchis CD, Risitano G, Milone D. Effect of Weight Distribution on Knee Joint Temperature Pattern Under Fatigue Condition. Engineering Proceedings. 2025; 85(1):43. https://doi.org/10.3390/engproc2025085043
Chicago/Turabian StyleSpataro, Marta, Davide Crisafulli, Cristiano De Marchis, Giacomo Risitano, and Dario Milone. 2025. "Effect of Weight Distribution on Knee Joint Temperature Pattern Under Fatigue Condition" Engineering Proceedings 85, no. 1: 43. https://doi.org/10.3390/engproc2025085043
APA StyleSpataro, M., Crisafulli, D., Marchis, C. D., Risitano, G., & Milone, D. (2025). Effect of Weight Distribution on Knee Joint Temperature Pattern Under Fatigue Condition. Engineering Proceedings, 85(1), 43. https://doi.org/10.3390/engproc2025085043






