Detection of Alzheimer’s and Parkinson’s Diseases Using Deep Learning-Based Various Transformers Models †
Abstract
1. Introduction
2. Related Works
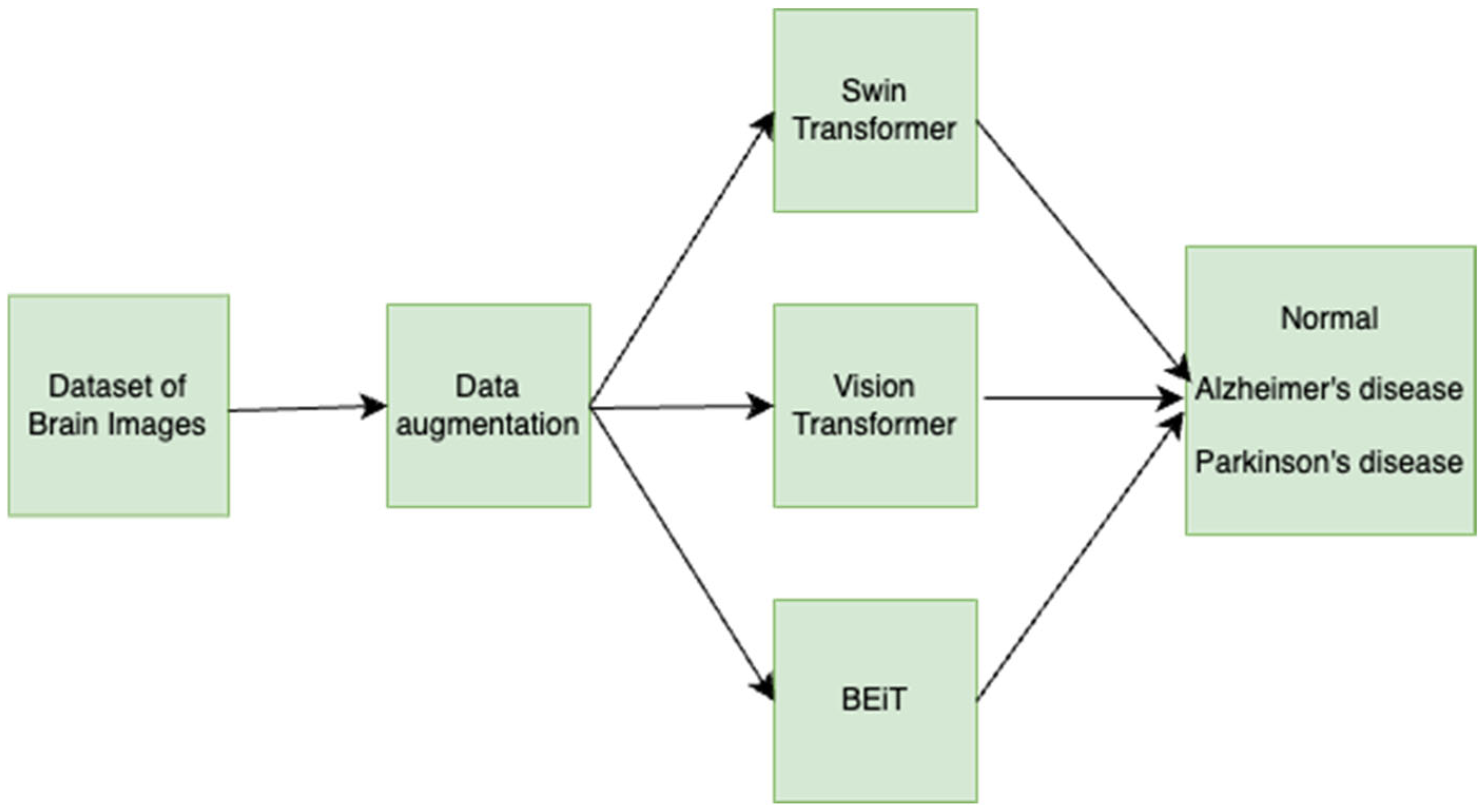
3. Materials and Methods
4. Results
5. Conclusions and Future Works
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alzheimer’s Disease. Available online: https://www.mayoclinic.org/diseases-conditions/alzheimers-disease/symptoms-causes/syc-20350447 (accessed on 7 May 2024).
- Parkinson’s Disease. Available online: https://www.mayoclinic.org/diseases-conditions/parkinsons-disease/symptoms-causes/syc-20376055 (accessed on 7 May 2024).
- Balaji, P.; Chaurasia, M.A.; Bilfaqih, S.M.; Muniasamy, A.; Alsid, L.E.G. Hybridized Deep Learning Approach for Detecting Alzheimer’s Disease. Biomedicines 2023, 11, 149. [Google Scholar] [CrossRef] [PubMed]
- El-Latif, A.A.A.; Chelloug, S.A.; Alabdulhafith, M.; Hammad, M. Accurate Detection of Alzheimer’s Disease Using Lightweight Deep Learning Model on MRI Data. Diagnostics 2023, 13, 1216. [Google Scholar] [CrossRef] [PubMed]
- Saratxaga, C.L.; Moya, I.; Picón, A.; Acosta, M.; Moreno-Fernandez-de-Leceta, A.; Garrote, E.; Bereciartua-Perez, A. MRI Deep Learning-Based Solution for Alzheimer’s Disease Prediction. J. Pers. Med. 2021, 11, 902. [Google Scholar] [CrossRef] [PubMed]
- Battineni, G.; Hossain, M.A.; Chintalapudi, N.; Traini, E.; Dhulipalla, V.R.; Ramasamy, M.; Amenta, F. Improved Alzheimer’s Disease Detection by MRI Using Multimodal Machine Learning Algorithms. Diagnostics 2021, 11, 2103. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, R.; Ghnemat, R.; Abu Al-Haija, Q. Improving Alzheimer’s Disease and Brain Tumor Detection Using Deep Learning with Particle Swarm Optimization. AI 2023, 4, 551–573. [Google Scholar] [CrossRef]
- Baydargil, H.B.; Park, J.-S.; Kang, D.-Y. Anomaly Analysis of Alzheimer’s Disease in PET Images Using an Unsupervised Adversarial Deep Learning Model. Appl. Sci. 2021, 11, 2187. [Google Scholar] [CrossRef]
- Hazarika, R.A.; Maji, A.K.; Kandar, D.; Jasinska, E.; Krejci, P.; Leonowicz, Z.; Jasinski, M. An Approach for Classification of Alzheimer’s Disease Using Deep Neural Network and Brain Magnetic Resonance Imaging (MRI). Electronics 2023, 12, 676. [Google Scholar] [CrossRef]
- Chintalapudi, N.; Battineni, G.; Hossain, M.A.; Amenta, F. Cascaded Deep Learning Frameworks in Contribution to the Detection of Parkinson’s Disease. Bioengineering 2022, 9, 116. [Google Scholar] [CrossRef] [PubMed]
- Maskeliūnas, R.; Damaševičius, R.; Kulikajevas, A.; Padervinskis, E.; Pribuišis, K.; Uloza, V. A Hybrid U-Lossian Deep Learning Network for Screening and Evaluating Parkinson’s Disease. Appl. Sci. 2022, 12, 11601. [Google Scholar] [CrossRef]
- Carvajal-Castaño, H.A.; Pérez-Toro, P.A.; Orozco-Arroyave, J.R. Classification of Parkinson’s Disease Patients—A Deep Learning Strategy. Electronics 2022, 11, 2684. [Google Scholar] [CrossRef]
- Elshewey, A.M.; Shams, M.Y.; El-Rashidy, N.; Elhady, A.M.; Shohieb, S.M.; Tarek, Z. Bayesian Optimization with Support Vector Machine Model for Parkinson Disease Classification. Sensors 2023, 23, 2085. [Google Scholar] [CrossRef] [PubMed]
- Guven, M.; Uysal, F. A New Method for Heart Disease Detection: Long Short-Term Feature Extraction from Heart Sound Data. Sensors 2023, 23, 5835. [Google Scholar] [CrossRef] [PubMed]
- Özdaş, M.B.; Uysal, F.; Hardalaç, F. Classification of Retinal Diseases in Optical Coherence Tomography Images Using Artificial Intelligence and Firefly Algorithm. Diagnostics 2023, 13, 433. [Google Scholar] [CrossRef] [PubMed]
- Uysal, F.; Köse, M.M. Classification of Breast Cancer Ultrasound Images with Deep Learning-Based Models. Eng. Proc. 2023, 31, 8. [Google Scholar] [CrossRef]
- Güven, M.; Hardalaç, F.; Özışık, K.; Tuna, F. Heart Diseases Diagnose via Mobile Application. Appl. Sci. 2021, 11, 2430. [Google Scholar] [CrossRef]
- Özdaş, M.B.; Uysal, F.; Hardalaç, F. Super Resolution Image Acquisition for Object Detection in the Military Industry. In Proceedings of the 5th International Congress on Human-Computer Interaction, Optimization and Robotic Applications, Ankara, Türkiye, 8–10 June 2023. [Google Scholar]
- Uysal, F.; Erkan, M. Multiclass Classification of Brain Tumors with Various Deep Learning Models. Eng. Proc. 2022, 27, 30. [Google Scholar] [CrossRef]
- Peker, O.; Uysal, F.; Hardalaç, F. Boost Loss Functions for Better Change Detection. In Proceedings of the 3rd International Informatics and Software Engineering Conference, Ankara, Türkiye, 15–16 December 2022. [Google Scholar]
- Uysal, F.; Erkan, M. Evrişimsel Sinir Ağları Temelli Derin Öğrenme Modelleri Kullanılarak Beyin Tümörü Manyetik Rezonans Görüntülerinin Sınıflandırılması. EMO Bilimsel Dergi 2023, 13, 19–27. [Google Scholar]
- Kaggle. Available online: https://www.kaggle.com/datasets/farjanakabirsamanta/alzheimer-diseases-3-class (accessed on 7 May 2024).
- Liu, Z.; Lin, Y.; Cao, Y.; Hu, H.; Wei, Y.; Zhang, Z.; Lin, S.; Guo, B. Swin transformer: Hierarchical vision transformer using shifted windows. In Proceedings of the IEEE/CVF International Conference on Computer Vision, Virtual, 11–17 October 2021. [Google Scholar]
- Dosovitskiy, A.; Beyer, L.; Kolesnikov, A.; Weissenborn, D.; Zhai, X.; Unterthiner, T.; Dehghani, M.; Minderer, M.; Heigold, G.; Gelly, S.; et al. An image is worth 16x16 words: Transformers for image recognition at scale. In Proceedings of the International Conference on Learning Representations, Virtual, 4 May 2021. [Google Scholar]
- Bao, H.; Dong, L.; Piao, S.; Wei, F. Beit: Bert pre-training of image transformers. In Proceedings of the International Conference on Learning Representations, Virtual, 25 April 2022. [Google Scholar]
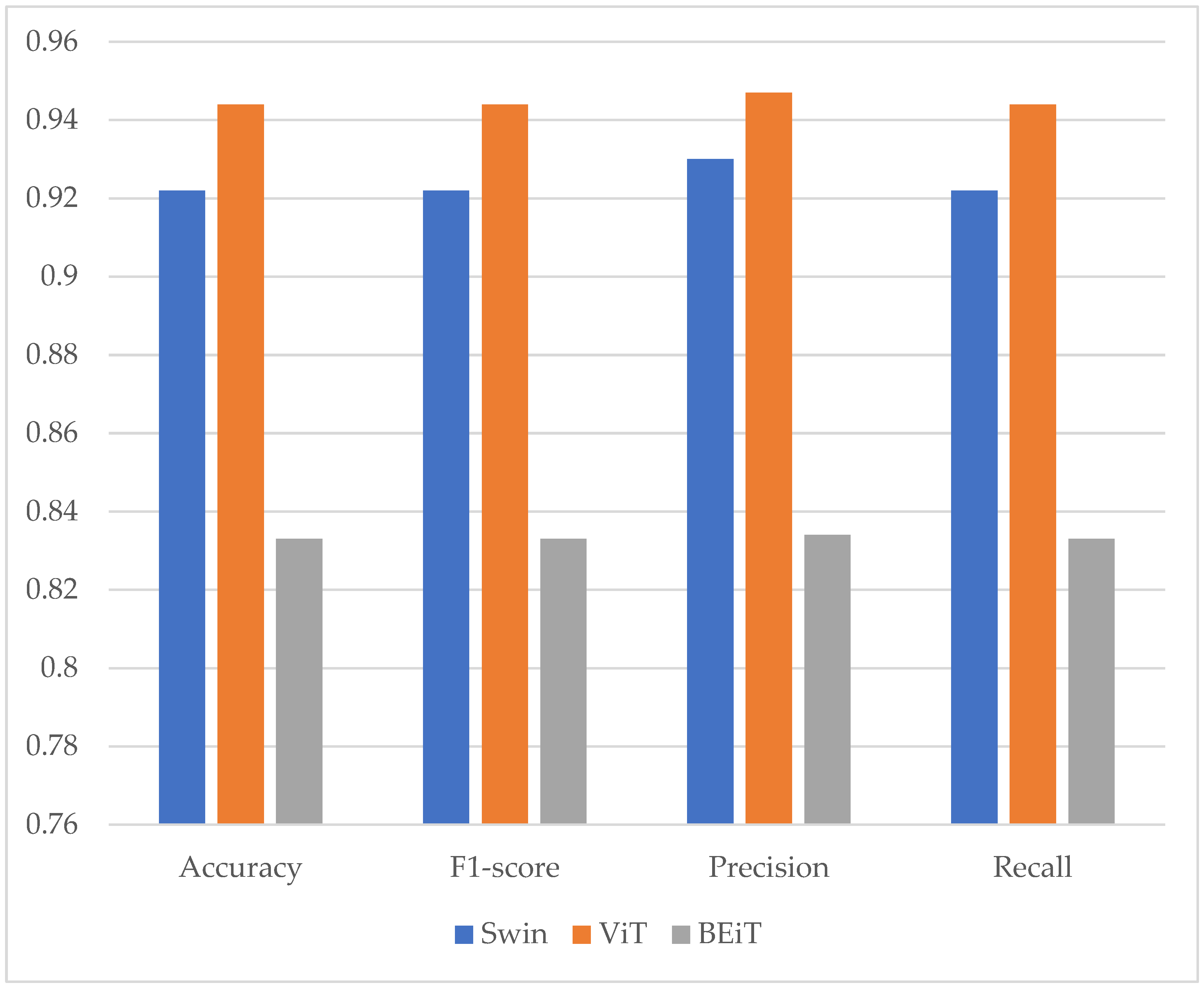
| Transformer Models | Loss | Accuracy | F1-Score | Precision | Recall |
|---|---|---|---|---|---|
| Swin | 0.216 | 0.922 | 0.922 | 0.930 | 0.922 |
| ViT | 0.134 | 0.944 | 0.944 | 0.947 | 0.944 |
| BEiT | 0.349 | 0.833 | 0.833 | 0.834 | 0.833 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Güven, M. Detection of Alzheimer’s and Parkinson’s Diseases Using Deep Learning-Based Various Transformers Models. Eng. Proc. 2024, 73, 4. https://doi.org/10.3390/engproc2024073004
Güven M. Detection of Alzheimer’s and Parkinson’s Diseases Using Deep Learning-Based Various Transformers Models. Engineering Proceedings. 2024; 73(1):4. https://doi.org/10.3390/engproc2024073004
Chicago/Turabian StyleGüven, Mesut. 2024. "Detection of Alzheimer’s and Parkinson’s Diseases Using Deep Learning-Based Various Transformers Models" Engineering Proceedings 73, no. 1: 4. https://doi.org/10.3390/engproc2024073004
APA StyleGüven, M. (2024). Detection of Alzheimer’s and Parkinson’s Diseases Using Deep Learning-Based Various Transformers Models. Engineering Proceedings, 73(1), 4. https://doi.org/10.3390/engproc2024073004






