1. Introduction
In 2020, a new variant of coronavirus spread around the world, known as SARS-CoV-2. This variant, which causes the COVID-19 pathology, is known to cause a viral pneumonia and severe acute respiratory syndrome as its main symptoms. Due to the aerosol transmission capabilities of the virus and the possibility of contagion through surfaces, specific protocols and independent circuits were designed in the health services in order to avoid cross-contamination between hospital personnel and patients. Chest X-rays and computerized tomography scans are mainly used to diagnose this pathology in order to determine the degree of the affliction of the patients, which allows us to see the state of the lungs in a non-invasive way. However, this medical imaging equipment is usually set up in rooms specifically designed for them, with certain safety measures. For this reason, to prevent said cross-contamination, the use of portable X-ray devices that can be used in these alternative circuits is recommended. On the other hand, these devices only allow a limited range of planes from which images of the patient can be extracted. Moreover, due to their nature, the images tend to be of lesser quality. These two factors, together with the emergency situation and the inherent subjectivity of a human expert, can result in challenges in making a quick, correct and repeatable diagnosis for further monitoring of the afflicted. It is precisely for this reason that the use of computer-based diagnostic support systems to assist in the task is necessary.
The main problem that emerged in the development of these methodologies derives from the scarcity of available samples due to the exceptionality of the scenario as well as the target domain. For this reason, methodologies were developed based on the prominent classical lung radiographs from fixed devices [
1,
2]. Even so, the results from these automatic methodologies were not as accurate as would be desired, as they were unprepared to work with these portable devices. This way, there were attempts to develop both methodologies trained with a reduced dataset with networks robust to this data scarcity [
3], methodologies that proposed generating synthetic samples from zero to train more powerful networks weak to this data scarcity [
4] and, like in the work proposed here, methodologies that aim to assist both clinicians and other computer-aided diagnosis systems by reducing the presence of extraneous elements [
5]: a robust lung segmentation strategy for chest radiographs from portable devices.
2. Materials and Methods
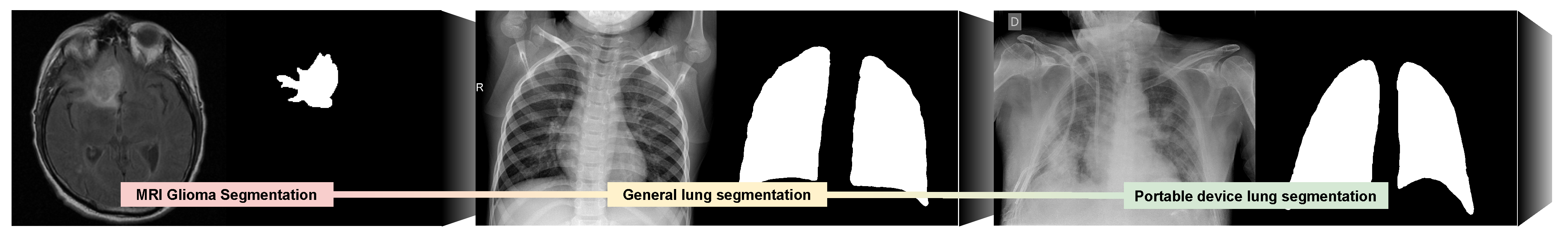
For the development of our proposal we employed three different domains represented in
Figure 1. Using as baseline brain magnetic resonance images for glioma segmentation, we took advantage of a pretrained U-Net model from the work of
Buda [
6]. These pathological bodies (and also natural structures present in the image) show similar gradient and texture patterns as lung regions afflicted by different respiratory tract diseases. The second domain consists in chest radiographs that were obtained with classical X-ray devices [
7,
8] to further approximate the deep features of the network to the target images from portable devices (introducing it to the patterns of the target organ and pathology). Finally, the third (and target) domain is composed of images that were captured during live clinical practice from a local hospital during the COVID-19 pandemic (the Universitary Hospital Complex of A Coruña or CHUAC, by its acronym in Spanish) with portable chest X-ray devices. To ensure that the system would be able to properly perform in a real clinical scenario in even the most borderline cases, both chest radiography datasets include both COVID-19 and healthy patients, but also a third class of pathological lung radiographs with a similar profile as patients with COVID-19 (but not being actually afflicted by it). These scenarios mainly include similar cases of viral and bacterial pneumonia that leave a very similar trace in the chest radiographs.
This way, we first adapt the classification layer of the U-Net pretrained with glioma images dataset and resume the training with the general lung radiographs. This allows the network to learn to segment these radiographs in a reduced number of epochs. Afterwards, we further refine the classification of this model by resuming the training, but now with images from our dataset composed by chest radiographs from portable devices.
3. Results and Discussion
The results attained in both transfer learning stages can be seen in
Table 1. In both cases, the results are shown with the same independent dataset with images that were extracted by means of portable X-ray devices. As we can see, the results that were obtained by the system are satisfactory with all the studied metrics. However, we see two metrics that clearly stand out from the rest after the second phase of transfer learning: the Dice and the sensitivity that improve by 0.0688 and 0.0804 on average in the three classes, respectively. These metrics indicate that, while in both cases the system was able to obtain an approximate segmentation to the lung region, after the second phase of knowledge transfer these segmentations are more adjusted to the regions of interest established by the experts (even despite the aforementioned deterioration in image quality and limitations). For this reason, we can see that, in fact, we have obtained a more robust system compared to those trained only with classical lung radiographs thanks to the progressive adaptation of the latent features of the network, and only needing a reduced number of samples.
Author Contributions
P.L.V.: Conceptualization, methodology, software, formal analysis, investigation, data curation, writing—original draft, writing—review and editing, visualization. J.d.M.: conceptualization, validation, investigation, data curation, writing—review and editing, supervision, project administration. J.N.: validation, investigation, data curation, writing—review and editing, supervision, project administration, funding acquisition. M.O.: validation, investigation, data curation, writing—review and editing, supervision, project administration, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
Instituto de Salud Carlos III, Government of Spain, DTS18/00136 research project; Ministerio de Ciencia e Innovación y Universidades, Government of Spain, RTI2018-095894-B-I00 research project, Ayudas para la formación de profesorado universitario (FPU), grant ref. FPU18/02271; Ministerio de Ciencia e Innovación, Government of Spain through the research project with reference PID2019-108435RB-I00; Consellería de Cultura, Educación e Universidade, Xunta de Galicia, Grupos de Referencia Competitiva, grant ref. ED431C 2020/24 and through the postdoctoral grant contract ref. ED481B 2021/059; Axencia Galega de Innovación (GAIN), Xunta de Galicia, grant ref. IN845D 2020/38; CITIC, Centro de Investigación de Galicia ref. ED431G 2019/01, receives financial support from Consellería de Educación, Universidade e Formación Profesional, Xunta de Galicia, through the ERDF (80%) and Secretaría Xeral de Universidades (20%).
Institutional Review Board Statement
The study was approved by the Ethics Review Board y Data Management Technical Commission of Galician Health Ministry for High Impact studies with protocol code 2020-007.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Ozturk, T.; Talo, M.; Yildirim, E.A.; Baloglu, U.B.; Yildirim, O.; Acharya, U.R. Automated detection of COVID-19 cases using deep neural networks with X-ray images. Comput. Biol. Med. 2020, 121, 103792. [Google Scholar] [CrossRef] [PubMed]
- de Moura, J.; Novo, J.; Ortega, M. Fully automatic deep convolutional approaches for the analysis of Covid-19 using chest X-ray images. IEEE Access 2020, 8, 195594–195607. [Google Scholar] [CrossRef]
- de Moura, J.; Garcia, L.R.; Vidal, P.L.; Cruz, M.; Lopez, L.A.; Lopez, E.C.; Novo, J.; Ortega, M. Deep Convolutional Approaches for the Analysis of COVID-19 Using Chest X-Ray Images From Portable Devices. IEEE Access 2020, 8, 195594–195607. [Google Scholar] [CrossRef]
- Moris, D.I.; de Moura, J.; Novo, J.; Ortega, M. Cycle Generative Adversarial Network Approaches to Produce Novel Portable Chest X-Rays Images for Covid-19 Diagnosis. In Proceedings of the ICASSP 2021—2021 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), Toronto, ON, Canada, 6–11 June 2021. [Google Scholar] [CrossRef]
- Vidal, P.L.; de Moura, J.; Novo, J.; Ortega, M. Multi-stage transfer learning for lung segmentation using portable X-ray devices for patients with COVID-19. Expert Syst. Appl. 2021, 173, 114677. [Google Scholar] [CrossRef] [PubMed]
- Buda, M. U-Net for Brain MRI, Pytorch Hub. 2020. Available online: https://pytorch.org/hub/mateuszbuda_brain-segmentation-pytorch_unet/ (accessed on 20 October 2020).
- Kermany, D. Labeled Optical Coherence Tomography (OCT) and Chest X-Ray Images for Classification. Mendeley Data 2018. Available online: https://data.mendeley.com/datasets/rscbjbr9sj/2 (accessed on 1 September 2020). [CrossRef]
- Cohen, J.P.; Morrison, P.; Dao, L.; Roth, K.; Duong, T.Q.; Ghassemi, M. COVID-19 Image Data Collection: Prospective Predictions Are the Future. arXiv 2020, arXiv:2006.11988. [Google Scholar]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).