Abstract
The ligand named 1-(3-chlorophenyl)-N-(pyridine-2-yl)methanimine Schiff base was obtained with the method of mixing 2-amino pyridine with 3-chloro benzaldehyde in methanol as an initial material. Transition metals, like Cu(II), were added to the prepared ligand as a dopant. The molecular structure was characterized by elemental analysis, electronic methods (UV-Visible), Vibrational methods (FT-IR), and 1H NMR spectroscopy. The catalytic activities of the complex were studied using Claisen–Schmidt condensation for the synthesis of chalcone derivatives employed using three different catalysts by an ultrasonication method. The results reveal that the Cu(II) complex showed remarkable catalytic activity and good yield compared to the other catalysts.
1. Introduction
Ligands are of great importance, and Schiff bases in particular have a significant role to play in coordination chemistry and coordinate with metal ions through azomethine nitrogen [1]. Metal complexes are prepared by the condensation reaction between aldehydes and amines with various transition metals. Despite extensive research in this area, these compounds remain highly relevant and interesting in the field of inorganic chemistry [2,3,4]. These compounds have been studied in a comprehensive way due to their remarkable chemical characteristics and their potential applications in diverse fields. Their relatively easy synthesis also adds to their appeal as research leads [5,6,7,8,9,10].
Schiff-base ligands have gained substantial attention in the coordination chemistry field owing to their simple synthesis, accessibility, and electrical characteristics. They have also been the focus of recent attention due to their importance in various fields [11]. They find extensive use in the catalysis and pharmaceutical industries [12,13].
This study intended to prepare metal complexes of Cu(II) with the Schiff bases obtained from 2-amino pyridine and 3-Chloro benzaldehyde. The prepared ligands and their metal complexes were evaluated using various analytical techniques like elemental analysis, electronic spectra, IR, and 1H NMR spectroscopic studies. Finally, we observed the synthesized complexes to be promising candidates to catalyze Claisen–Schmidt condensation for the synthesis of chalcone derivatives (bioactive substances) with good yield.
2. Experimental Methods
2.1. Materials and Methods
Highly pure chemicals of analytical reagent grade (AR) were utilized. They included 2- amino pyridine (Sigma-Aldrich, Chennai, India), 3- Chloro benzaldehyde (Sigma-Aldrich, Chennai, India), and CuSO4·2H2O (Sigma-Aldrich, Chennai, India). Throughout the experiment, double-distilled water was utilized.
2.2. Instruments
An Elementar Vario EL III analyzer was utilized to conduct chemical analyses on carbon, hydrogen, nitrogen, and chlorine elements. KBr discs were used to record FT-IR spectra on a Perkin-Elmer 1650 spectrometer, New York, NY, USA within the range of 4000–400 cm−1. The 1H NMR spectra were obtained using TMS as an internal standard and recorded using a 300 MHz Varian-Oxford Mercury instrument Palo Alto, CA, USA as a solution in DMSO-d6. The UV-Visible spectra were recorded in the range of 200–1000 nm on a JASCO V770 spectrometer, Easton, PA, USA.
2.3. Synthesis of Schiff-Base Ligand—1-(3-Chlorophenyl)-N-(pyridine-2-yl)methanimine [CPPM]
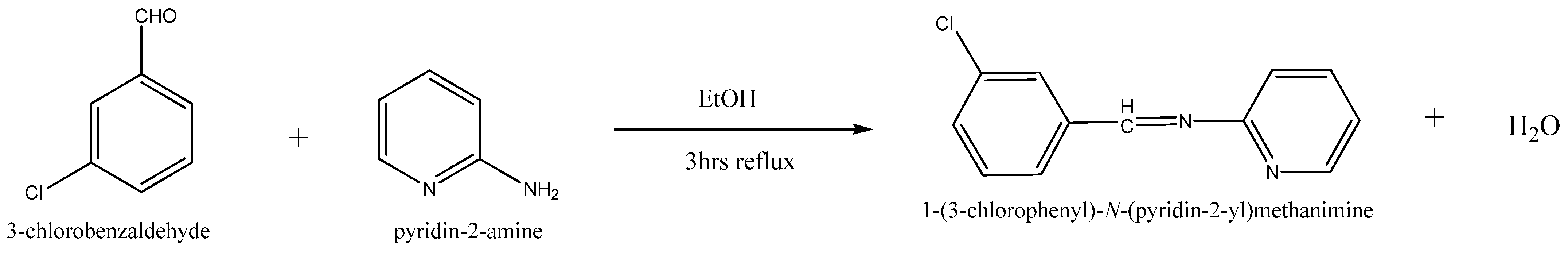
Equimolar concentrations of 2-amino pyridine (0.05 mol, 4.70 g) with 3- Chloro benzaldehyde (0.05 mol, 5.66 mL) in ethanol were used as a solvent. Glacial acetic acid (2 drops) was introduced to the content. The same was warmed up to room temperature and then agitated for approximately 4 h under reflux. A pale yellowish solid compound was isolated after cooling. After filtering the precipitate and washing it with ice-cold water, the residue was recondensed from methanol. The new compound was synthesized by the method summarized in Scheme 1.
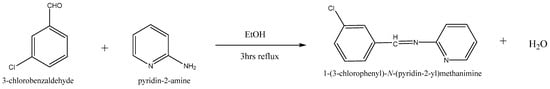
Scheme 1.
Synthesis of Schiff-base ligand (CPPM).
2.4. Synthesis of Schiff-Base Metal Complexes
The preparation of the metal complexes was performed through the addition of the appropriate metal sulphate, CuSO4 (0.5 g, 0.002 mol), in water to the solution of the ligand (1 g, 0.004 mol) in a mixture of ethanol and a drop of glacial acetic acid was introduced to the content. The resultant mixture was agitated under reflux for five hours. A bluish-green metal complex was obtained after filtering and subsequent washing, followed by drying.
3. Results and Discussion
3.1. Elemental Analysis
Table 1 contains information regarding the ligand and its metal complexes, including information obtained from analysis and specific physical characteristics. The metal complexes had distinct colors and were not prone to absorbing moisture. Based on the analysis, the metal complexes exhibited a 2:1 ratio of ligand to metal. The observed percentages of carbon, hydrogen, nitrogen, and metal ion aligned well with the values expected, confirming the proposed structural formula, [M(CPPM)2], where CPPM is 1-(3-chlorophenyl)-N-(pyridine-2-yl)methanimine.

Table 1.
Elemental analysis data of the ligand (CPPM) and its corresponding metal complexes.
3.2. Electronic Spectral Study
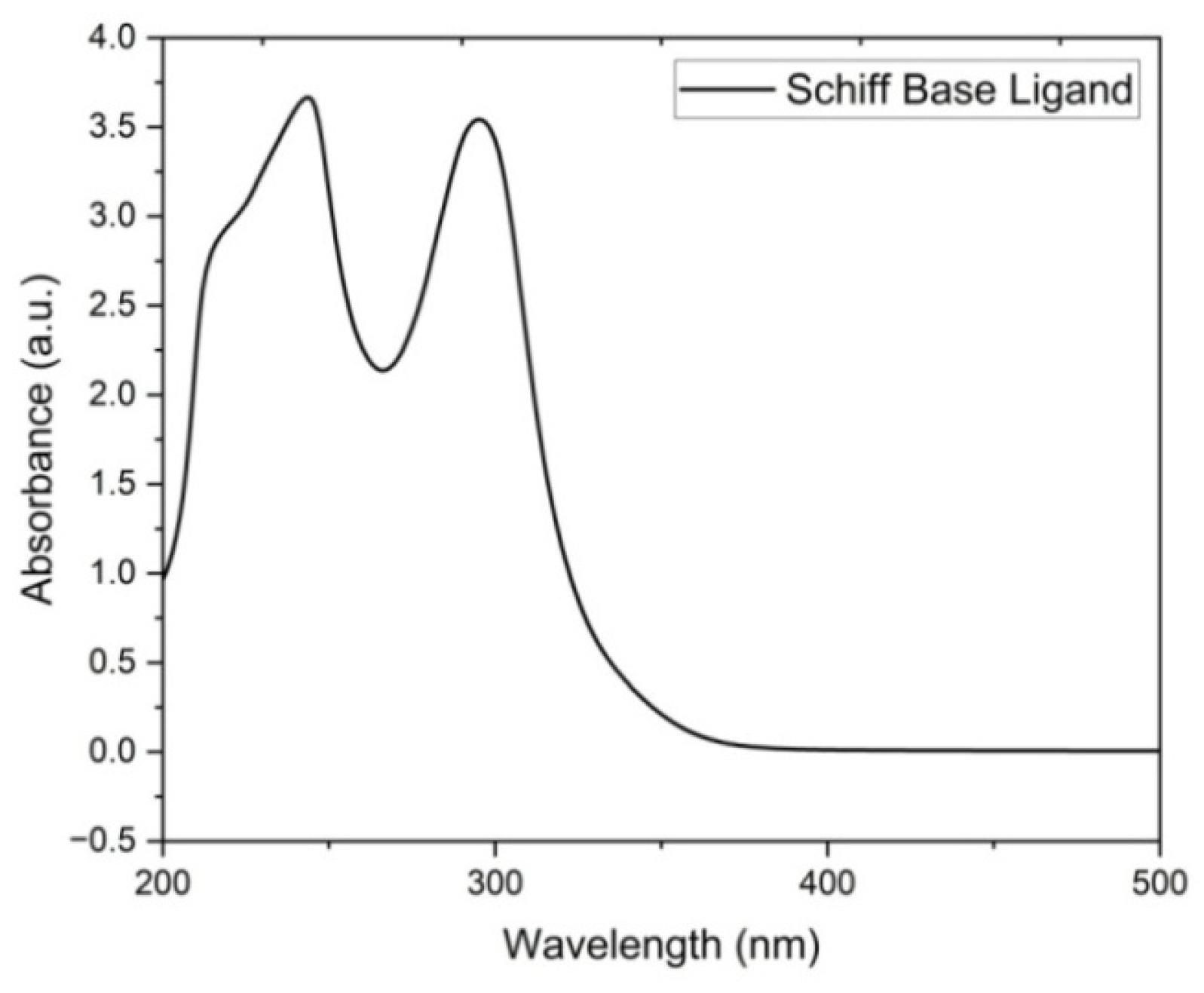
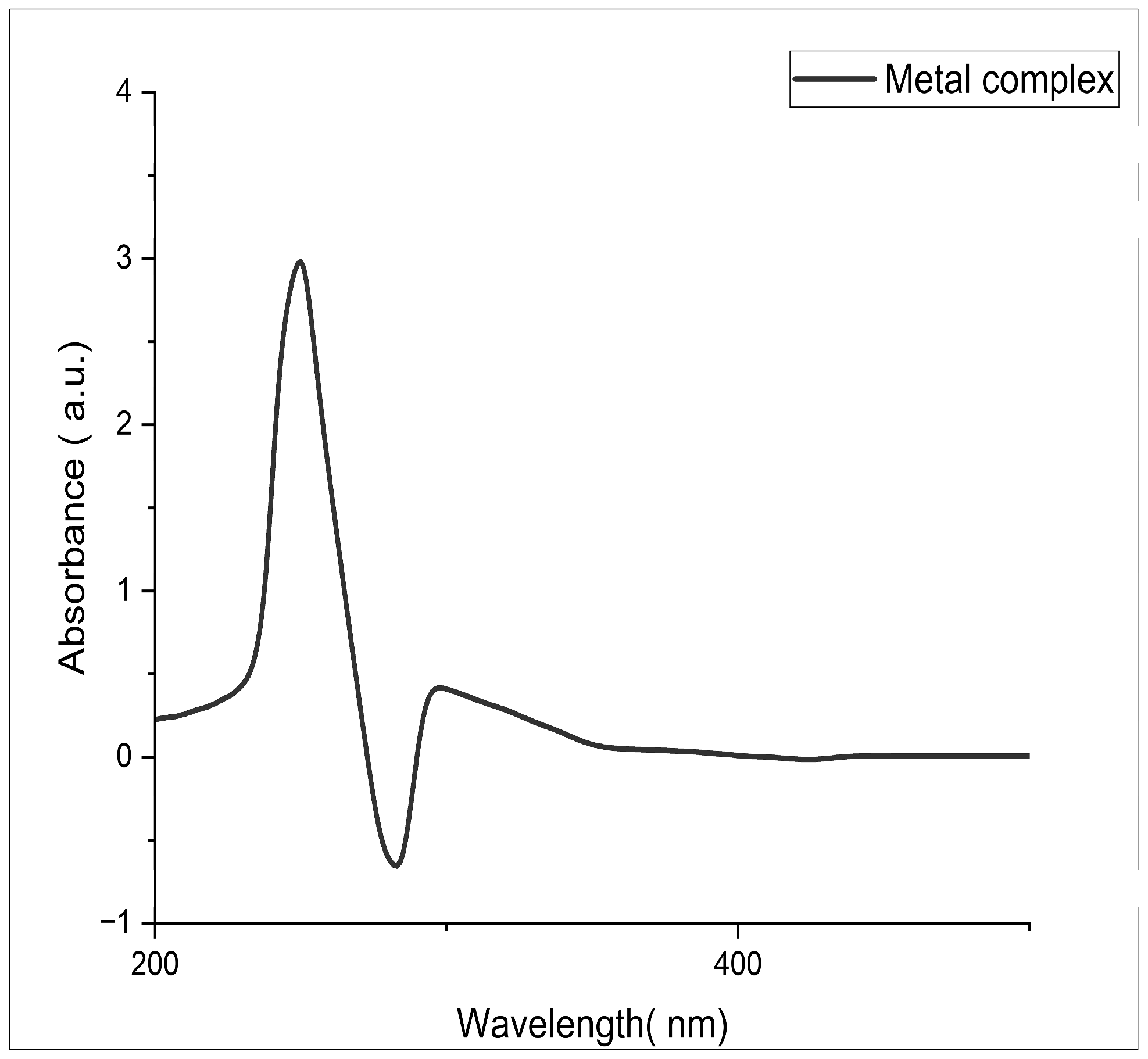
Figure 1 shows the absorption spectra of the ligand 1-(3-chlorophenyl)-N-(pyridine-2-yl)methanimine and exhibits characteristic bands at 212.63, 231.27, and 311.67 nm. The absorption bands at 212.63 and 231.27 nm correspond to the π–π* transition, while the peak at 311.67 nm indicates the appearance of the n–π* transition. The changes in electronic energy levels suggest that the ligand contains an azomethine group [14,15]. Figure 2 shows the absorption spectra of the complex and it is noted that the copper complexes exhibit a distinct absorption band between 276.68 and 313.77 nm, which indicates the formation of a complex ion. Data pertaining to the spectral properties are given in Table 2.
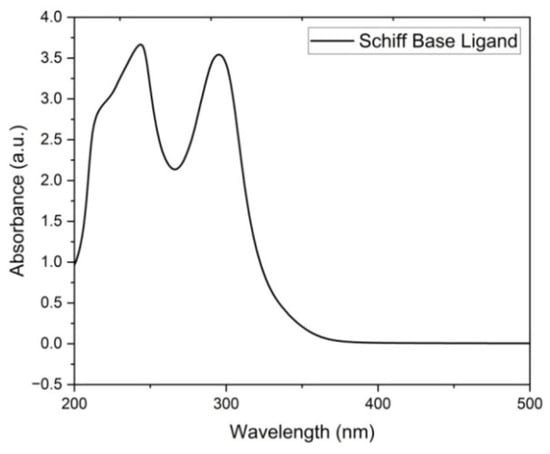
Figure 1.
Electronic spectrum of Schiff-base ligand 1-(3-chlorophenyl)-N-(pyridine-2-yl)methanimine.
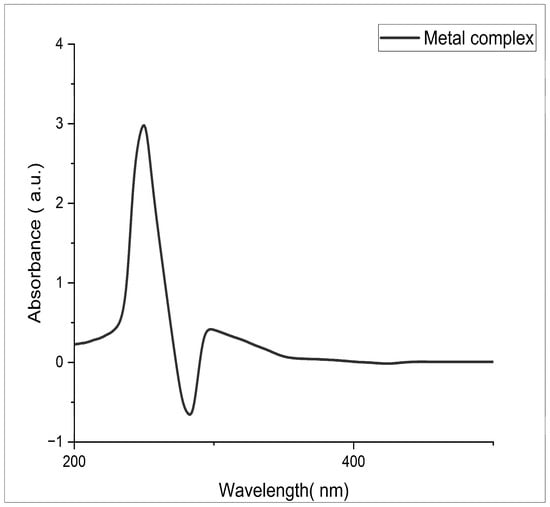
Figure 2.
Electronic spectrum of Schiff-base Cu(II) complex.

Table 2.
Electronic spectral data of Schiff-base ligand (CPPM) and its corresponding metal complexes.
3.3. IR Spectroscopic Study
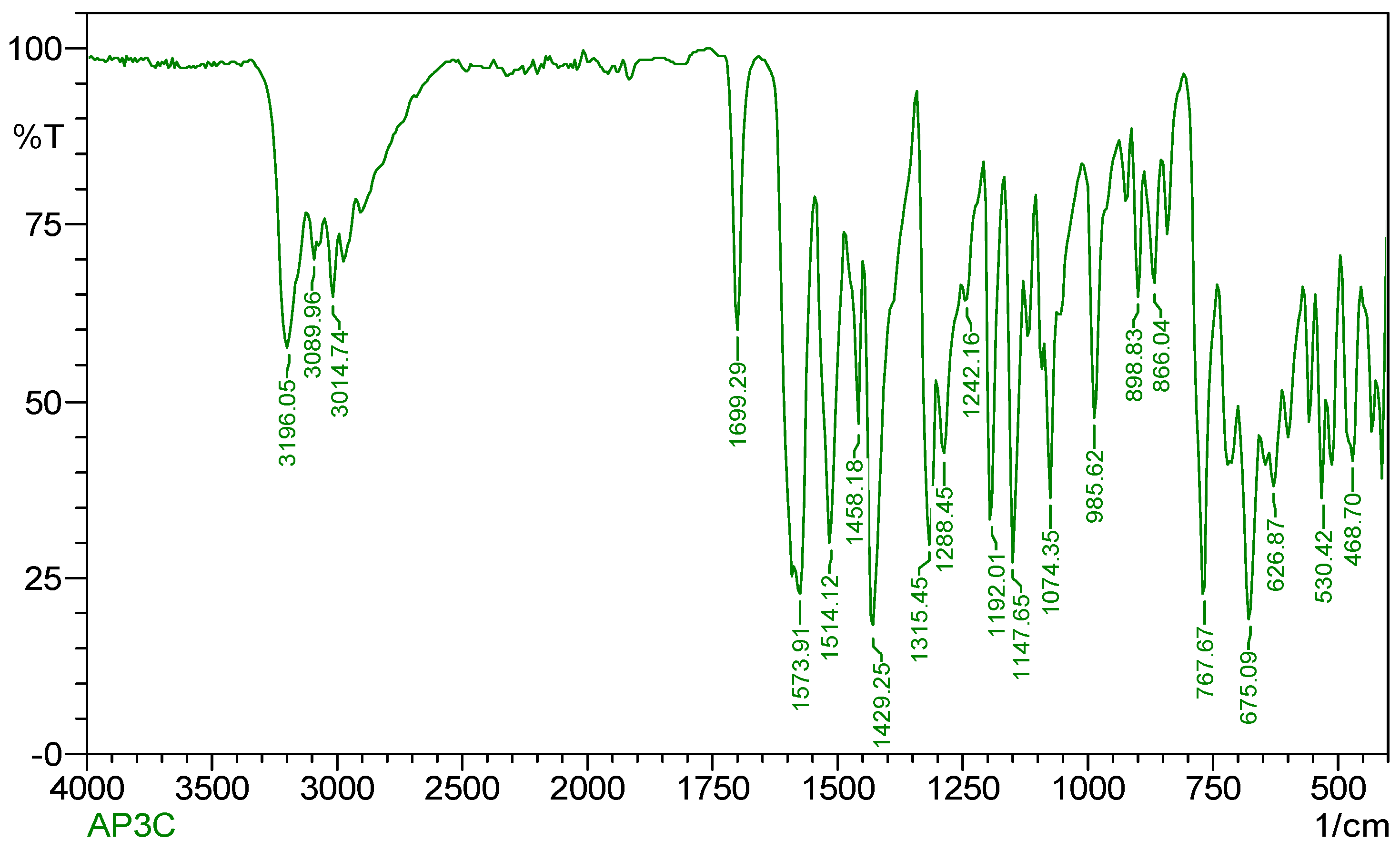
Figure 3 indicates the IR spectra of the ligand (CPPM) which shows a moderately intense band at 1699 cm−1 and it is ascribed to the ν(C=N) vibration of the azomethine group [16]. A band in the 1434 cm−1 region shows the occurrence of an aromatic ring, while in Schiff-base complexes, its presence is confirmed by a band appearing in the 1667 cm−1 region, which corresponds to the ν(C=N) band. IR spectral data of Cu(II) complex of the ligand CPPM are given in Table 3.
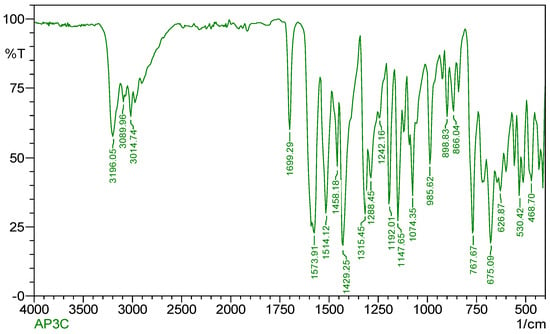
Figure 3.
IR spectrum of Schiff--base ligand.

Table 3.
IR spectral data of ligand (CPPM) and its corresponding complexes (cm−1).
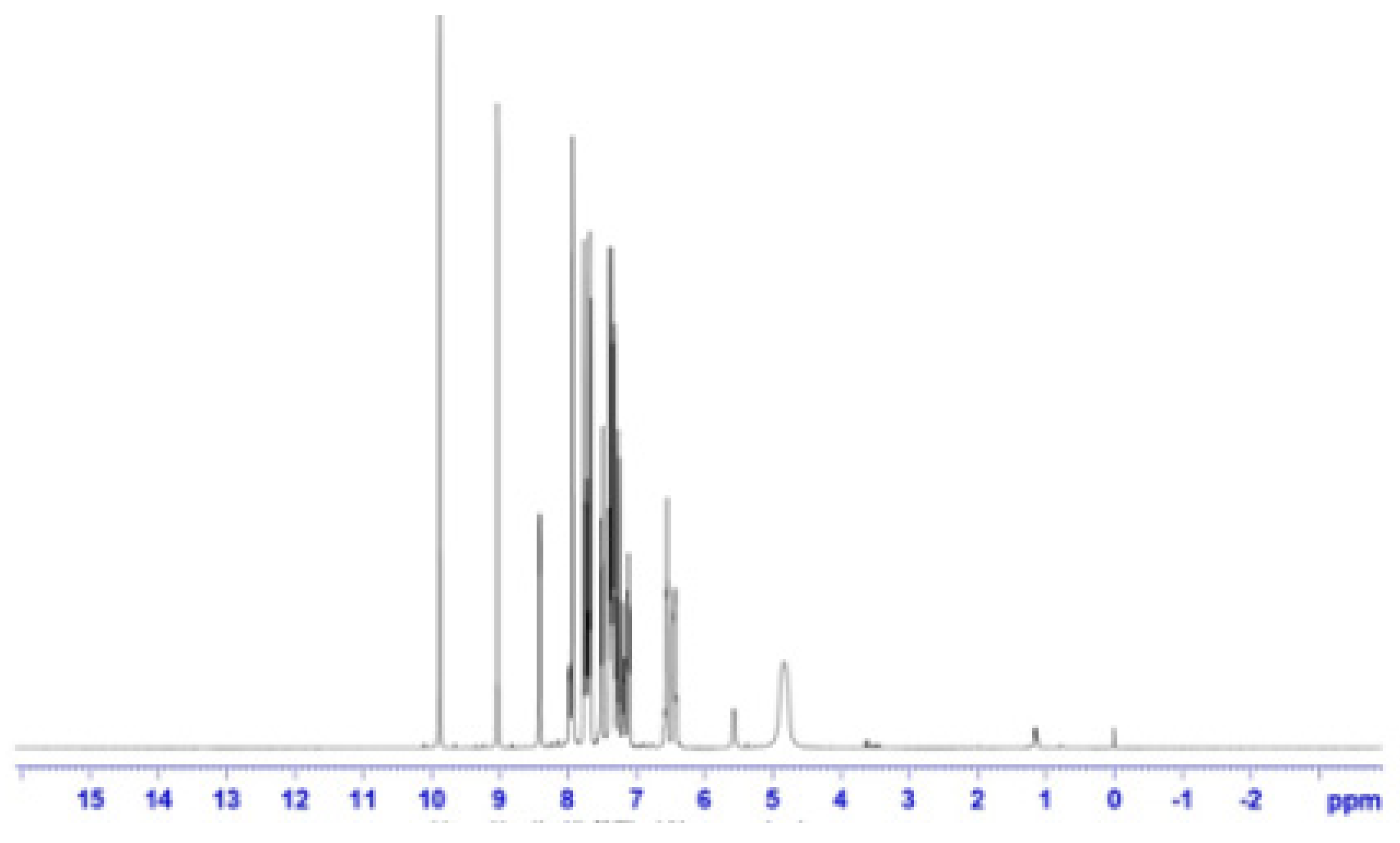
3.4. 1HNMR Spectral Analysis
The presence of the azomethine proton was indicated by two peaks in the 8.2–8.8 ppm range. A series of multiplets between 6.58 and 7.7 ppm was attributed to the aromatic protons. The results align with conclusions derived from the analysis of the IR spectrum (Figure 4).
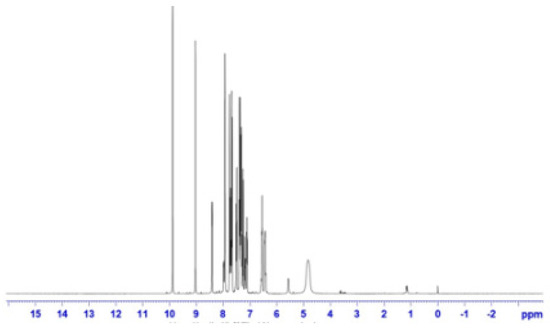
Figure 4.
1HNMR spectrum of Schiff-base ligand.
4. Catalytic Studies
In this study, Claisen–Schmidt condensation was used for the synthesis of chalcone derivatives. The condensation of various aldehydes (Benzaldehyde (0.00942 mol), Salicyaldehyde (0.00819 mol), and Anisaldehyde (0.00734 mol)) was treated with acetophenone in the presence of three different catalysts, including KOH (0.000178 mol), p-Toluene sulphonic acid (PTSA) (0.00005807 mol), and the metal complex (1.959 mol). The same was sonicated for 60 min in an ultrasonic cleaner water bath at 70 °C. The contents were subsequently cooled, filtered, and dried, followed by recrystallization from alcohol to produce chalcone.
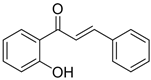
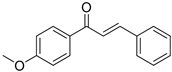
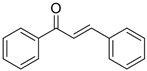
Table 4 demonstrates that chalcones were successfully synthesized with a high yield by condensing acetophenone with various types of aromatic aldehydes under ultrasound irradiation at 70 °C for 60 min, with a metal complex acting as a catalyst. The results of this method were compared to those of two other catalysts. The current method resulted in a greater product yield and shorter reaction time when compared to previously reported procedures [17,18,19,20] in the literature. From the above data, it is evident that Schiff-base metal complexes were used as efficient catalysts with an appreciable yield of >90%.

Table 4.
Reaction of various aldehydes with acetophenone in the presence of different catalysts.
The physicochemical and IR data of synthesized compounds catalyzed by the Cu (II) complex are shown in Table 5.

Table 5.
Physicochemical and IR data of synthesized compounds catalyzed by Cu (II) complex.
5. Conclusions
The complexation of a metal with a Schiff-base ligand was carried out and analyzed using elemental analysis, UV-Visible, IR, and 1H NMR techniques. The prepared complex was used as a catalyst in the production of chalcone derivatives through ultrasonication. The results indicate that the prepared complex had a noteworthy ability to catalyze a reaction, with a higher yield (more than 90%) compared to other catalysts such as KOH and PTSA. Based on the above study, it can be inferred that the prepared metal complexes can be used as potential catalysts for organic synthesis.
Author Contributions
Conceptualization, G.B. and K.K.; methodology, A.D., G.B., K.K., K.S.R. and A.R.; formal analysis, A.D. and G.B.; writing—original draft preparation, G.B. and R.K.; writing—review and editing, K.S.R. and A.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to the fact that this work is part of ongoing research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vigato, P.A.; Tamburini, S. The Challenge of Cyclic and Acyclic Schiff Bases and Related Derivatives. Coord. Chem. Rev. 2004, 248, 1717–2128. [Google Scholar] [CrossRef]
- Patel, N.H.; Parekh, H.M.; Patel, M.N. Synthesis, characterization and biological evaluation of manganese (II), cobalt (II), nickel (II), copper (II), and cadmium (II) complexes with mono basic (NO) and neutral (NN) Schiff bases. Transit. Met. Chem. 2005, 30, 13–17. [Google Scholar] [CrossRef]
- Thakor, Y.J.; Patel, S.G.; Patel, K.N. Synthesis, Characterization and biocidal studies of some transition metal complexes containing tetra dentate and neutral bi dentate Schiff base. J. Chem. Pharm. Res. 2010, 2, 518–525. [Google Scholar]
- Ramesh, R.; Suganthy, P.K.; Natarajan, K. Synthesis, spectra and electrochemistry of Ru(III) complexes with tetradentate Schiff bases. Synth. React. Inorg. Met.-Org. Chem. 1996, 26, 47–60. [Google Scholar] [CrossRef]
- Enis, N.M.Y.; Thahira, S.A.; Edward, R.T.; Abhimanyu, V.; Karen, A.C.; Mohamed, I.M.; Haslina, A. Synthesis, characterization and Biological Evaluation of Transition Metal Complexes Derived from N, S Bidentate Ligands. Int. J. Mol. Sci. 2015, 16, 11034–11054. [Google Scholar]
- Pedreño, E.; López-Contreras, A.J.; Cremades, A.; Peñafiel, R. Protecting or promoting effects of spermine on DNA strand breakage induced by iron or copper ions as a function of metal Concentration. J. Inorg. Biochem. 2005, 99, 2074–2080. [Google Scholar] [CrossRef] [PubMed]
- Suja, P.S.; Theodore, S.; Antony, R.; Muthupoongodi, S.; Sathyasheeli, S. New class of Copper (II) complex derived from Isatin and Thiosemicarbazide—Synthesis, Spectral Characterization and biological activity. Pharma Chem. 2016, 8, 67–76. [Google Scholar]
- Yoon, T.P.; Jacobsen, E.N. Privileged Chiral Catalysts. Science 2003, 299, 1691. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-G, J.M.; Lopez-Duran, F.A.; Hernandez-Ortega, S.; Gomez-Vidales, V.; Macias- Ruvalcaba, N.; Aguilar-Martínez, M. The structures and cyclic voltammetry of three copper (II) complexes derived from bulky ortho-hydroxy Schiff bases. J. Mol. Struct. 2002, 612, 69–79. [Google Scholar] [CrossRef]
- AKhandar, A.; Nejati, K. Synthesis and characterization of a series of copper (II) complexes with azo-linked salicylaldehyde Schiff base ligands. Polyhedron 2000, 19, 607–613. [Google Scholar] [CrossRef]
- Boulechfar, C.; Ferkous, H.; Delimi, A.; Djedouani, A.; Kahlouche, A.; Boublia, A.; Darwish, A.S.; Lemaoui, T.; Verma, R.; Benguerba, Y. Schiff bases and their metal Complexes: A review on the history, synthesis, and applications. Inorg. Chem. Commun. 2023, 150, 110451. [Google Scholar] [CrossRef]
- Gaur, S. Physico-chemical and Biological Properties of Mn(II), Co(II), Ni(II) and Cu(II) Chelates of Schiff Bases. Asian J. Chem. 2003, 15, 250–254. [Google Scholar]
- Gemi, M.J.; Biles, C.; Keiser, B.J.; Poppe, S.M.; Swaney, S.M.; Tarapley, W.G.; Romeso, D.L.; Yage, Y. Novel 1,5-diphenylpyrazole nonnucleoside HIV-1 reverse transcriptase inhibitors with enhanced activity versus the delavirdine-resistant P236L mutant: Lead identification and SAR of 3- and 4-substituted derivatives. J. Med. Chem. 2000, 43, 1034–1040. [Google Scholar]
- Lekha, L.; Raja, K.K.; Rajagopal, G.; Easwaramoorthy, D. Synthesis, spectroscopic characterization and antibacterial studies lanthanide (III) Schiff base complexes containing N, O donor atoms. J. Mol. Struct. 2014, 1056, 307–313. [Google Scholar] [CrossRef]
- Chohan, Z.H.; Supuran, C.T.; Scozzafava, A. Metalloantibiotics: Synthesis and Antibacterial Activity of Cobalt (II), Copper (II), Nickel (II) and Zinc (II) Complexes of Kefzol. J. Enzym. Inhib. Med. Chem. 2004, 19, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Rajagopal, G.; Athappan, P.R. Synthesis, spectral and redox properties of metal complexes of macrocyclic tetraaza chiral Schiff bases. Trans. Met. Chem. 2001, 26, 588–593. [Google Scholar] [CrossRef]
- Toda, F.; Tanaka, K.; Hamai, K. Aldol condensations in the absence of solvent: Acceleration of the reaction and enhancement of the stereoselectivity. J. Chem. Soc. Perkin Trans. 1 1990, 3207–3209. [Google Scholar] [CrossRef]
- Doshi, A.G.; Ghiya, B.J. Synthesis and Antimicrobial Properties of Flavone Imines. Curr. Sci. 1986, 55, 502–503. [Google Scholar]
- Alcantara, A.R.; Marinas, J.M.; Sinisterra, J.V. Synthesis of 2′-hydroxychalcones and related compounds in interfacial solid-liquid conditions. Tetrahedron Lett. 1987, 28, 1515–1518. [Google Scholar] [CrossRef]
- Li, J.; Yang, W.; Wang, S.; Li, S.; Li, T. Improved synthesis of chalcones under ultrasound irradiation. Ultrason. Sonochem. 2002, 9, 237–239. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).