Abstract
The change in levels of volatile organic compounds (VOC) present in exhaled breath can be indicative of bodily disorders. Detection of such low levels of VOCs can allow early detection and diagnosis of diseases. A polymer- modified Quartz Tuning Fork (QTF) is a promising, cost-effective sensor that can detect a change in ppm levels of VOCs exhaled from the breath at room temperature. Acetone and acetaldehyde are biomarkers that are readily exhaled by human beings. Increased levels of these analytes can serve as indicators for toxicity or a wide array of diseases. The present work uses an array of QTFs modified separately using nanomaterials embedded in polystyrene to detect low VOC concentrations present in simulated human breath successfully. The sensor response shows a clear distinction between healthy human breath and breath spiked with varying VOC concentrations (5–400 ppm). The sensor response proves it can potentially serve as an economical and non-invasive tool for disease diagnostics.
1. Introduction
Human beings exhale numerous volatile organic compounds (VOCs) whose levels range in parts-per-million (ppm) or parts-per-billion (ppb). These VOCs may be of local, endogenous, or exogenous origins [1]. The origins can be traced to varied biochemical, biological, and cellular processes occurring in the body. VOCs are either subtracted from inspired air (by degradation and/or excretion in the body) or added to alveolar breath as products of metabolism. The air that is inhaled goes into the alveoli in the lungs where the metabolic excretable products diffuse into the inhaled air and then it is rejected in the form of exhaled air. Therefore, the exhaled air must carry the fingerprint of the endogenous metabolic processes and alterations in the levels of these exhaled compounds may serve as indicators or biomarkers of diseases.
Exhaled human breath is comprised mostly of nitrogen (78.04%), oxygen (16%), carbon dioxide (4–5%), hydrogen (5%), inert gases (0.9%), water vapor [2]. Different biochemical and physiological processes generate VOCs which can be classified as alcohols, aldehydes, acids, or ketones. For instance, inorganic VOCs such as nitric oxide (10–50 ppb), nitrous oxide (1–20 ppb), ammonia (0.5–2 ppm), carbon monoxide (0–6 ppm), hydrogen sulphide (0–1.3 ppm), etc., and organic VOCs such as acetone (0.3–1 ppm), ethanol, isoprene (∼105 ppb), ethane (0–10 ppb), methane (2–10 ppm), pentane [0–10 ppb], etc. are commonly exhaled. The research on breath analysis over the years has indicated the origin of numerous VOCs and linked them with specific diseases. The concentration of fractional exhaled nitric oxide has been reported for monitoring respiratory disorders such as asthma, chronic cough, and chronic obstructive pulmonary disorder [3]. Breath isoprene levels have been linked to the presence of chronic kidney disease, diabetes, blood cholesterol, lung cancer, and end-stage renal failure [4]. High levels of exhaled ammonia (>550 ppb) are associated with kidney malfunction such as chronic kidney disease [5]. Acetone and acetaldehyde are two such VOCs which are readily exhaled by humans and varying levels are associated with many diseases. Acetaldehyde level change corresponds to acute respiratory distress syndrome, lung cancer, chronic pulmonary disorder, and exposure to ethanol among others [6,7]. Acetone is a well-known biomarker of diabetes, lung cancer, and can indicate heart failure [6,8]. Thus, acetone and acetaldehyde were chosen for this study owing to the multitude of diagnostic possibilities using these two VOCs.
Measurement and analysis of levels and types of exhaled VOCs are carried out by a variety of techniques; gas-chromatography-mass-spectrometry (GC-MS), ion mobility spectrometry (MCC-IMS), proton transfer reaction mass spectrometry (PTR-MS), differential mobility spectrometer (DMS), etc. [9]. Gas sensors such as optical gas sensors [10], mass-sensitive gas sensors [11,12], chemiresisitve sensors [13], and SAW detectors [14] are reportedly used to identify type or concentration of VOC biomarkers. However, these techniques are expensive and often operate at high temperatures. Therefore, in this work, we propose the use of nanoparticle-enhanced polymer modified Quartz tuning forks as low concentration gas sensors.
Quartz tuning forks (QTFs) are single crystal quartz mechanical oscillators whose piezoelectric material quartz allows the QTF to be excited electrically and its resonant frequency to be read out electrically [15,16]. In recent years, QTFs have been widely used as sensors due to their high stability, precision, large Q factor, low power consumption and high thermal stability. QTFs are mechanical transducers, since changes in the mechanical properties of the system can be monitored by measuring the changes in resonant frequency which is measured in the form of an electrical signal. After modification, when a polymer film forms between the tines of a QTF and its resonant frequency increases, it is called a spring loaded system. Here, we have developed such QTFs modified with polymer films as sensors for detection of low concentrations of acetone and acetaldehyde spiked in human breath.
2. Materials and Methods
2.1. Materials
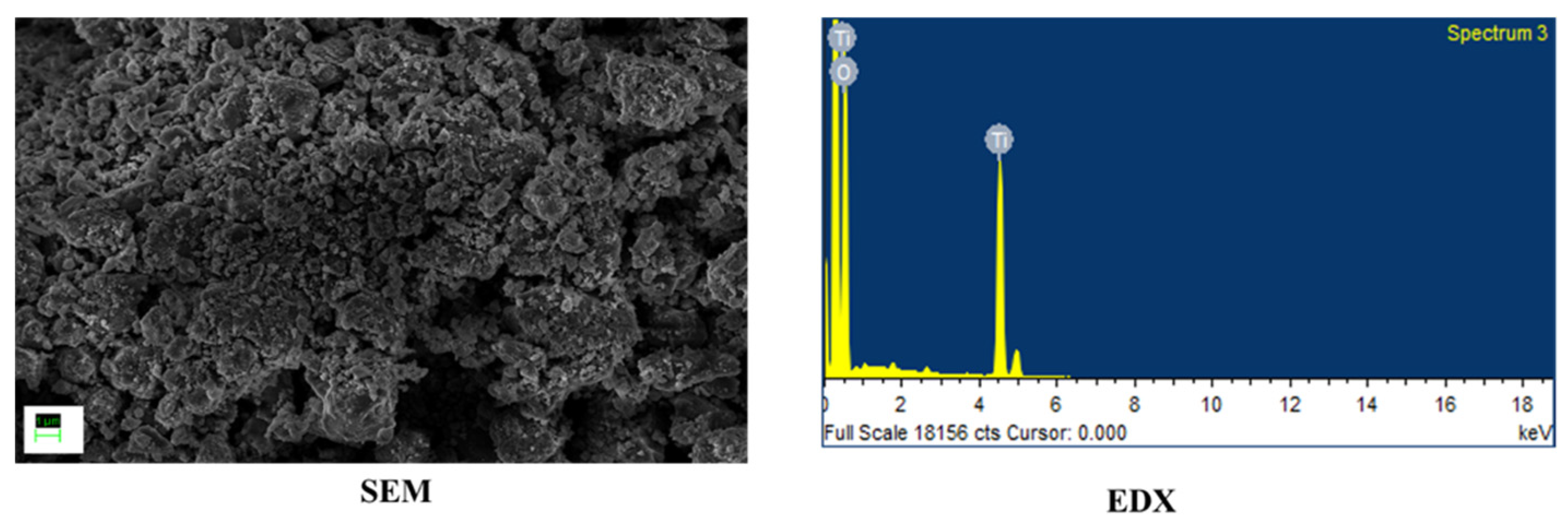
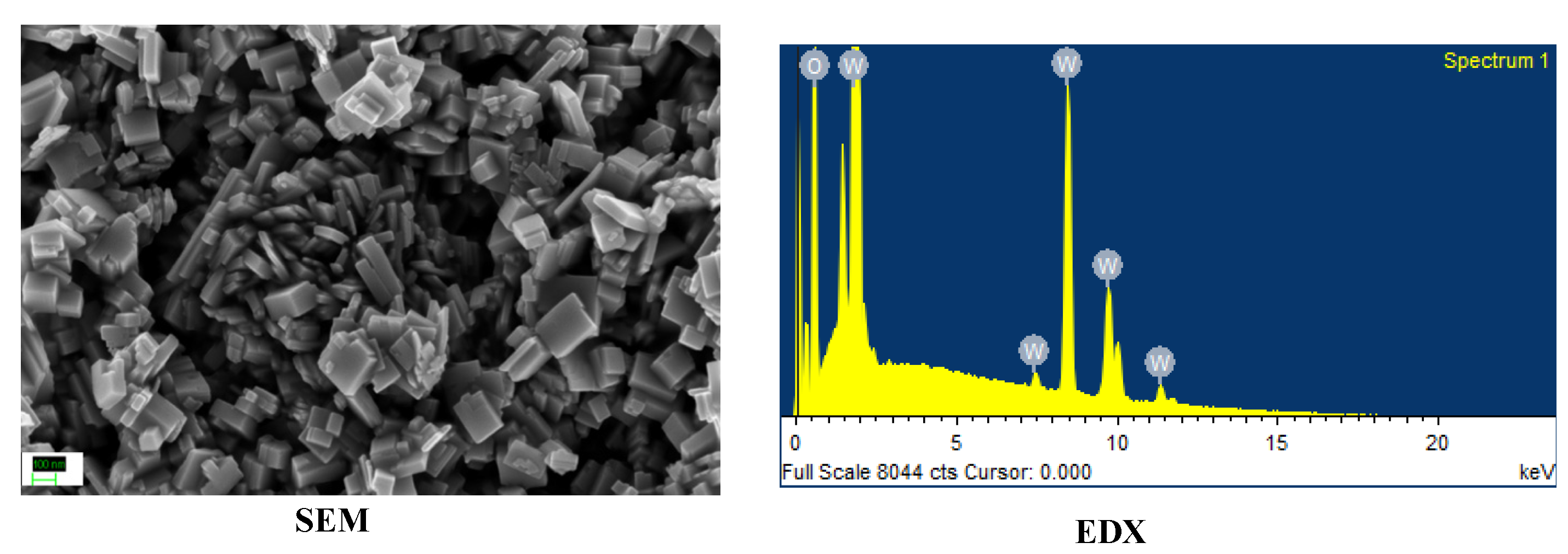
For this work, TiO2 and WO3 nanostructures were synthesized. All chemicals were used as procured. TiO2 nanoparticles were synthesized used a simple sol–gel procedure found elsewhere [17]. WO3 nanorods were synthesized by a hydrothermal method reported elsewhere [18]. The synthesized nanostructures were characterized by identifying the morphology using Scanning Electron Microscopy (SEM) and the elemental composition was confirmed using Energy Dispersive X-ray Analysis (EDX). Both Figure 1 and Figure 2 showed formation of nanosized structures over the area of study. The EDX graphs captured peaks corresponding to the elemental composition of the synthesized materials and a lack of other elements indicated an impurity-free composition.
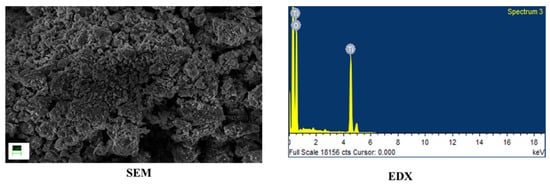
Figure 1.
Morphology and elemental composition of TiO2 nanoparticles.
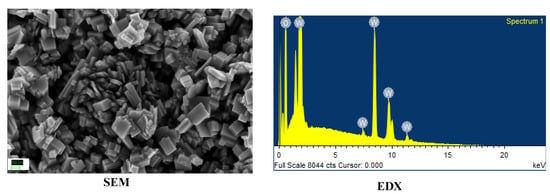
Figure 2.
Morphology and elemental composition of WO3 nanorods.
2.2. Modification of QTFs
For modification of the quartz tuning forks, a 5 weight% Polystyrene blend was prepared in aniline by stirring at room temperature. To the prepared solution, 1 weight% of the prepared nanoparticles was added and stirred for 24 h. Once a stable blend was obtained, 5 µL of the polymer/nanostructure solution was dropped into deionized water bath. Since the blend is hydrophobic in nature, a thin layer of polymer film is formed on the surface of water. A QTF is submerged under the water and taken out such that the as formed film is caught on the tines of the QTF. The resultant QTFs are dried for 24 h at room temperature.
3. Results
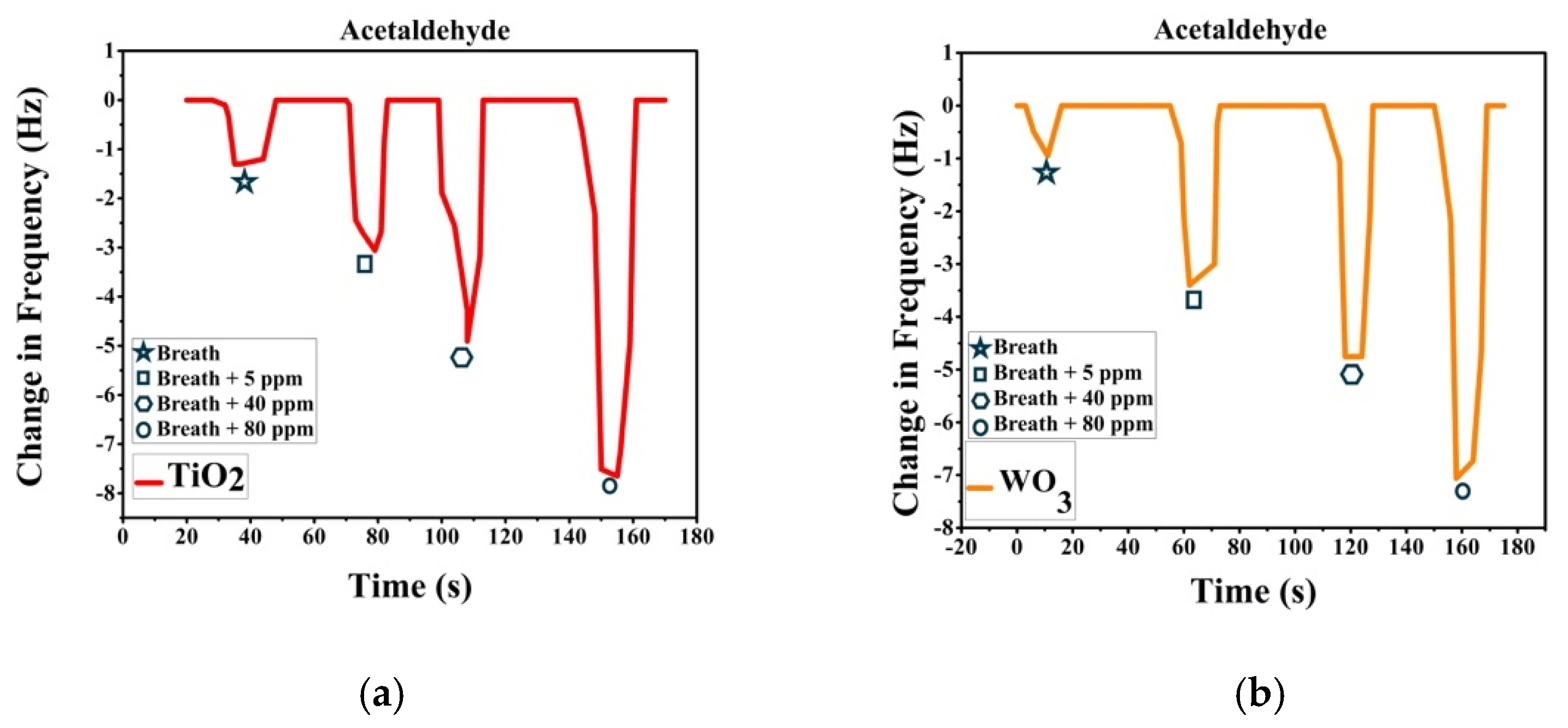
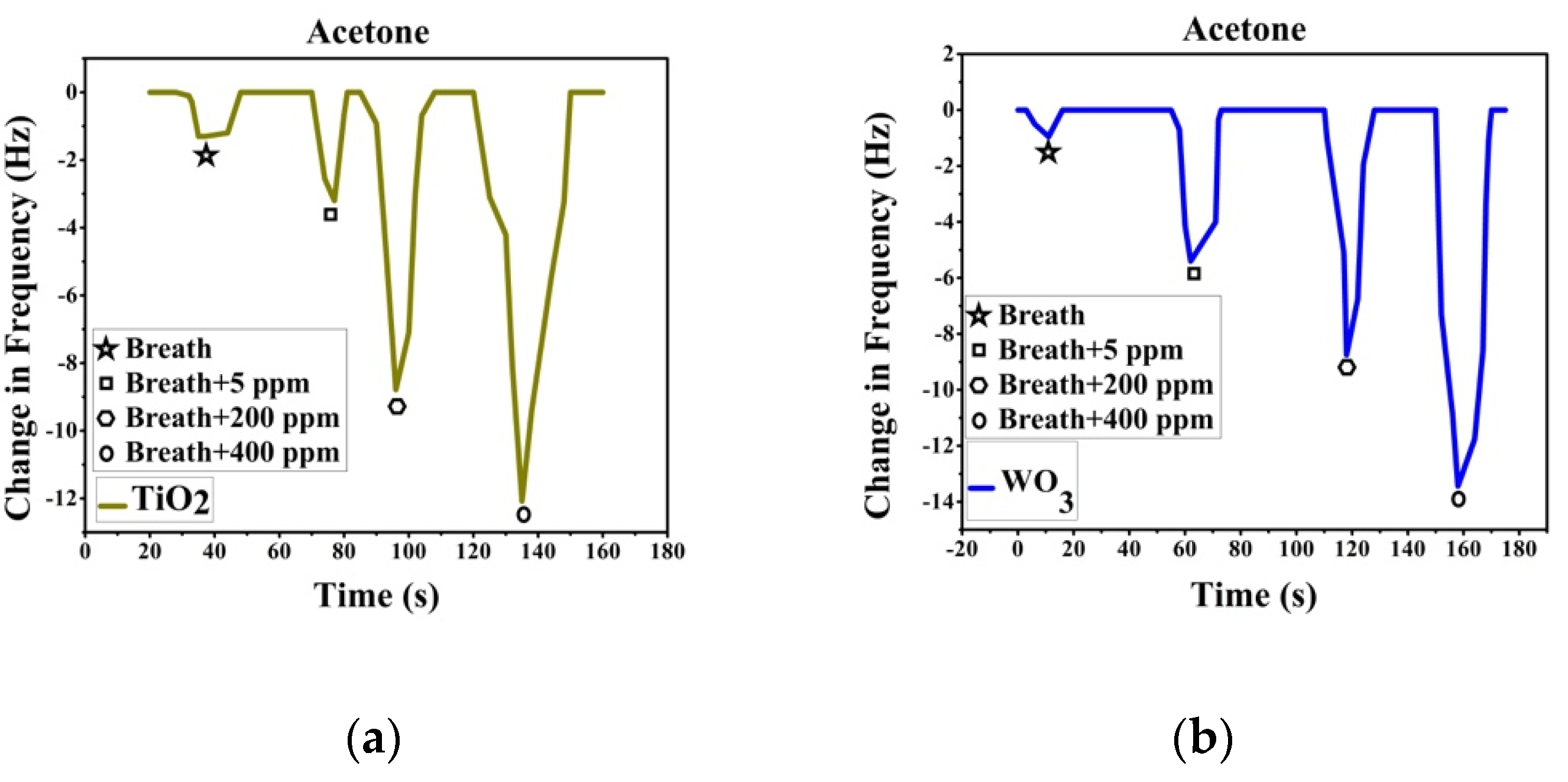
Sensor response was recorded after passing breath and VOC spiked breath over the sensor array. Breath was collected with ethical consent from volunteers. A known volume of VOC is added to the breath samples collected to make simulated breath which is then impinged on the sensors. The change in frequency is noted as the sensor response. Figure 3a shows the response recorded by TiO2-PS and response in Figure 3b is from WO3-PS modified QTFs to breath and acetaldehyde spiked breath (5–80 ppm). Figure 4a shows the response recorded by TiO2-PS and Figure 4b shows the response of WO3-PS modified QTFs to breath and acetone spiked breath (5–400 ppm).
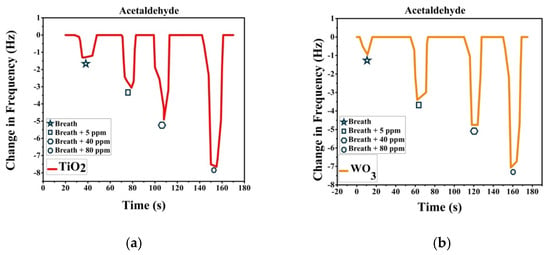
Figure 3.
Sensor response of pure and acetaldehyde-spiked breath for (a) TiO2-PS and (b) WO3-PS modified QTFs.
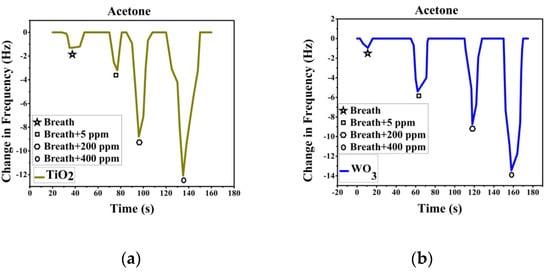
Figure 4.
Sensor response of pure and acetone-spiked breath for (a) TiO2 -PS and (b) WO3-PS modified QTFs.
Sensor response shows that both sensors give a comparable response to 5 ppm acetaldehyde spiked breath while WO3-PS gives a higher frequency change to 5 ppm acetone. The created sensors are capable of differentiating between varied ppm level concentrations of VOCs and provide a sufficient response to pure breath, thereby are suitable candidates for gas sensing.
4. Conclusions
TiO2-PS and WO3-PS modified QTFs served as low concentration VOC sensors. The sensors were able to distinguish between pure breath and VOC spiked breath even at 5 ppm concentration. WO3-PS modified QTFs gave a better sensor response to 5 ppm acetone while both WO3- and TiO2-PS gave similar response to 5 ppm acetaldehyde.
Author Contributions
Conceptualization, B.R. and S.D.; methodology, B.R.; software, B.R.; validation, B.R., S.P., S.D.; formal analysis, B.R., S.P., S.D.; investigation, B.R., S.M.D., S.P.; resources, B.R., S.M.D.; data curation, B.R., S.M.D., S.P.; writing—original draft preparation, B.R.; writing—review and editing, S.D.; supervision, S.D.; project administration, S.D.; funding acquisition, B.R., S.P., S.D. All authors have read and agreed to the published version of the manuscript.
Funding
B.R. received Senior Research Fellowship (5/3/8/61/ITR-F/2020-ITR) from Indian Council of Medical Research. S.P. received Senior Research Fellowship (09/992(0005)/2019/EMR-I) from Council of Scientific and Industrial Research.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- van der Schee, M.P.; Paff, T.; Brinkman, P.; van Aalderen, W.M.C.; Haarman, E.G.; Sterk, P.J. Breathomics in Lung Disease. Chest 2015, 147, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Pal, M. Non-invasive monitoring of human health by exhaled breath analysis: A comprehensive review. J. Electrochem. Soc. 2020, 167, 037562. [Google Scholar] [CrossRef]
- Guo, Y.; Hong, C.; Liu, Y.; Chen, H.; Huang, X.; Hong, M. Diagnostic value of fractional exhaled nitric oxide for asthma-chronic obstructive pulmonary disease overlap syndrome. Medicine 2018, 97, e10857. [Google Scholar] [CrossRef]
- Alkhouri, N.; Singh, T.; Alsabbagh, E.; Guirguis, J.; Chami, T.; Hanouneh, I.; Grove, D.; Lopez, R.; Dweik, R. Isoprene as a Potential Biomarker for Advanced Fibrosis. Clin. Transl. Gastroenterol. 2015, 6, e112. [Google Scholar] [CrossRef]
- Obermeier, J.; Trefz, P.; Happ, J.; Schubert, J.K.; Staude, H.; Fischer, D.C.; Miekisch, W. Exhaled volatile substances mirror clinical conditions in pediatric chronic kidney disease. PLoS ONE 2017, 12, e0178745. [Google Scholar]
- Jia, Z.; Patra, A.; Kutty, V.K.; Venkatesan, T. Critical review of volatile organic compound analysis in breath and in vitro cell culture for detection of lung cancer. Metabolites 2019, 9, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bos, L.D.; Weda, H.; Wang, Y.; Knobel, H.H.; Nijsen, T.M.; Vink, T.J.; Zwinderman, A.H.; Sterk, P.J.; Schultz, M.J. Exhaled breath metabolomics as a noninvasive diagnostic tool for acute respiratory distress syndrome. Eur. Respir. J. 2014, 44, 188–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcondes-Braga, F.G.; Gutz, I.G.; Batista, G.L.; Saldiva, P.H.; Ayub-Ferreira, S.M.; Issa, V.S.; Mangini, S.; Bocchi, E.A.; Bacal, F. Exhaled acetone as a new biomarker of heart failure severity. Chest 2012, 142, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, A.; Davidsen, J.R.; Titlestad, I.; Vestbo, J.; Baumbach, J. A systematic review of breath analysis and detection of volatile organic compounds in COPD. J. Breath Res. 2016, 10, 034002. [Google Scholar] [CrossRef] [PubMed]
- Iitani, K.; Chien, P.J.; Suzuki, T.; Toma, K.; Arakawa, T.; Iwasaki, Y.; Mitsubayashi, K. Fiber-optic bio-sniffer (biochemical gas sensor) using reverse reaction of alcohol dehydrogenase for exhaled acetaldehyde. ACS Sens. 2018, 3, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Debliquy, M.; Lahem, D.; Yan, Y.; Raskin, J.P. A Review on Functionalized Graphene Sensors for Detection of Ammonia. Sensors 2021, 21, 1443. [Google Scholar] [CrossRef] [PubMed]
- Wang, L. Metal-organic frameworks for QCM-based gas sensors: A review. Sens. Actuators A 2020, 307, 111984. [Google Scholar] [CrossRef]
- Tai, H.; Wang, S.; Duan, Z.; Jiang, Y. Evolution of breath analysis based on humidity and gas sensors: Potential and challenges. Sens. Actuators B 2020, 318, 128104. [Google Scholar] [CrossRef]
- Phillips, M.; Cataneo, R.N.; Cruz-Ramos, J.A.; Huston, J.; Ornelas, O.; Pappas, N.; Pathak, S. Prediction of breast cancer risk with volatile biomarkers in breath. Breast Cancer Res. Treat. 2018, 170, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Sampson, S.A.; Date, K.S.; Panchal, S.V.; Ambrale, A. and Datar, S.S. Investigation of QTF based gas sensors. Sens. Actuators B 2015, 216, 586–594. [Google Scholar] [CrossRef]
- Sampson, S.A.; Panchal, S.V.; Mishra, A.; Banerjee, S.; Datar, S.S. Quartz tuning fork based portable sensor for vapor phase detection of methanol adulteration of ethanol by using aniline-doped polystyrene microwires. Microchim. Acta 2017, 184, 1659–1667. [Google Scholar] [CrossRef]
- Mahshid, S.; Askari, M.; Ghamsari, M.S. Synthesis of TiO2 nanoparticles by hydrolysis and peptization of titanium isopropoxide solution. J. Mater. Process. Technol. 2007, 189, 296–300. [Google Scholar] [CrossRef]
- Tehrani, F.S.; Ahmadian, H.; Aliannezhadi, M. Hydrothermal synthesis and characterization of WO3 nanostructures: Effect of reaction time. Mater. Res. Express 2020, 7, 015911. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).