Microfabricated Gold Aptasensors for the Label-Free Electrochemical Assay of Oxytetracycline Residues in Milk †
Abstract
:1. Introduction
2. Experiment
2.1. Reagents and Materials
2.2. Instrumentation
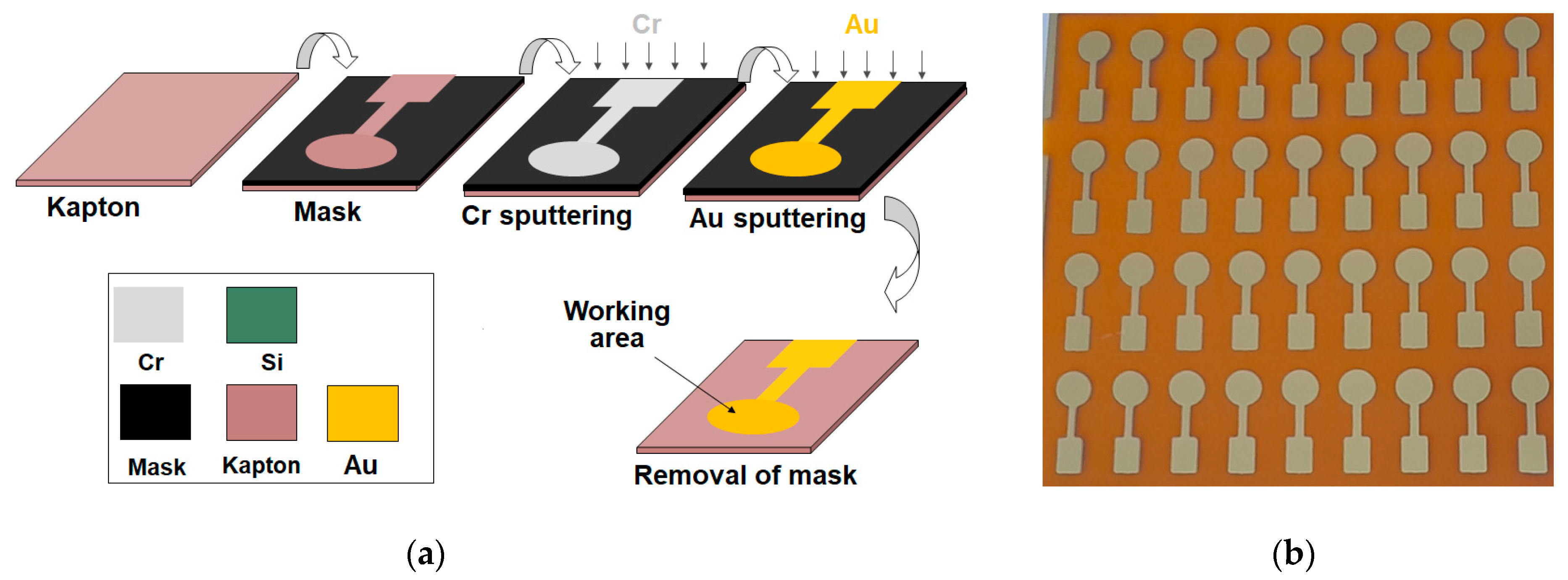
2.3. Fabrication of the Thin-Film Gold Sensors
2.4. Experimental Protocol
3. Results and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Landers, T.F.; Cohen, B.; Wittum, T.E.; Larson, E.L. A Review of Antibiotic Use in Food Animals: Perspective, Policy, and Potential. Public Health Rep. 2012, 127, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Virto, M.; Santamarina-García, G.; Amores, G.; Hernández, I. Antibiotics in Dairy Production: Where Is the Problem? Dairy 2022, 3, 541–564. [Google Scholar] [CrossRef]
- Commission Regulation (EU), No. 37/2010. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32010R0037 (accessed on 13 November 2023).
- Sversut, R.A.; da Silva, A.A.; Mazon Cardoso, T.F.; Kassab, N.M.; do Amaral, M.S.; Nunes Salgado, H.R. A Critical Review of Properties and Analytical Methods for the Determination of Oxytetracyline in Biological and Pharmaceutical Matrices. Crit. Rev. Anal. Chem. 2017, 47, 154–171. [Google Scholar] [CrossRef] [PubMed]
- Peris-Vicente, J.; Peris-García, E.; Albiol-Chiva, J.; Durgbanshi, A.; Ochoa-Aranda, E.; Carda-Broch, S.; Bose, D.; Esteve-Romero, J. Liquid chromatography, a valuable tool in the determination of antibiotics in biological, food and environmental samples. Microchem. J. 2022, 177, 107309. [Google Scholar] [CrossRef]
- Mehlhorn, A.; Rahimi, P.; Joseph, Y. Aptamer-Based Biosensors for Antibiotic Detection: A Review. Biosensors 2018, 8, 54. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Song, L.; Gao, Y.; Wu, K.; Guo, R.; Chen, R.; Zhen, J.; Pan, L. Aptamer Sensors for the Detection of Antibiotic Residues—A Mini-Review. Toxics 2023, 11, 513. [Google Scholar] [CrossRef] [PubMed]
- Evtugyn, G.; Porfireva, A.; Tsekenis, G.; Oravczova, V.; Hianik, T. Electrochemical Aptasensors for Antibiotics Detection: Recent Achievements and Applications for Monitoring Food Safety. Sensors 2022, 22, 3684. [Google Scholar] [CrossRef] [PubMed]
- Kejnovská, Ι.; Renčiuk, D.; Palacký, J.; Vorlíčková, M. CD Study of the G-Quadruplex Conformation. Methods Mol. Biol. 2019, 2035, 25–44. [Google Scholar] [CrossRef] [PubMed]




| [OTC] (μg/L) | ME |
|---|---|
| 25 | 1.25 |
| 50 | 1.23 |
| 100 | 1.15 |
| 200 | 1.15 |
| 400 | 1.11 |
| 600 | 1.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machairas, V.; Anagnostoupoulos, A.; Soulis, D.; Economou, A.; Jakab, K.; Melios, N.; Keresztes, Z.; Tsekenis, G.; Wang, J.; Speliotis, T. Microfabricated Gold Aptasensors for the Label-Free Electrochemical Assay of Oxytetracycline Residues in Milk. Eng. Proc. 2023, 58, 1. https://doi.org/10.3390/ecsa-10-16018
Machairas V, Anagnostoupoulos A, Soulis D, Economou A, Jakab K, Melios N, Keresztes Z, Tsekenis G, Wang J, Speliotis T. Microfabricated Gold Aptasensors for the Label-Free Electrochemical Assay of Oxytetracycline Residues in Milk. Engineering Proceedings. 2023; 58(1):1. https://doi.org/10.3390/ecsa-10-16018
Chicago/Turabian StyleMachairas, Vassilis, Andreas Anagnostoupoulos, Dionysios Soulis, Anastasios Economou, Kristóf Jakab, Nikitas Melios, Zsófia Keresztes, George Tsekenis, Joseph Wang, and Thanassis Speliotis. 2023. "Microfabricated Gold Aptasensors for the Label-Free Electrochemical Assay of Oxytetracycline Residues in Milk" Engineering Proceedings 58, no. 1: 1. https://doi.org/10.3390/ecsa-10-16018
APA StyleMachairas, V., Anagnostoupoulos, A., Soulis, D., Economou, A., Jakab, K., Melios, N., Keresztes, Z., Tsekenis, G., Wang, J., & Speliotis, T. (2023). Microfabricated Gold Aptasensors for the Label-Free Electrochemical Assay of Oxytetracycline Residues in Milk. Engineering Proceedings, 58(1), 1. https://doi.org/10.3390/ecsa-10-16018









