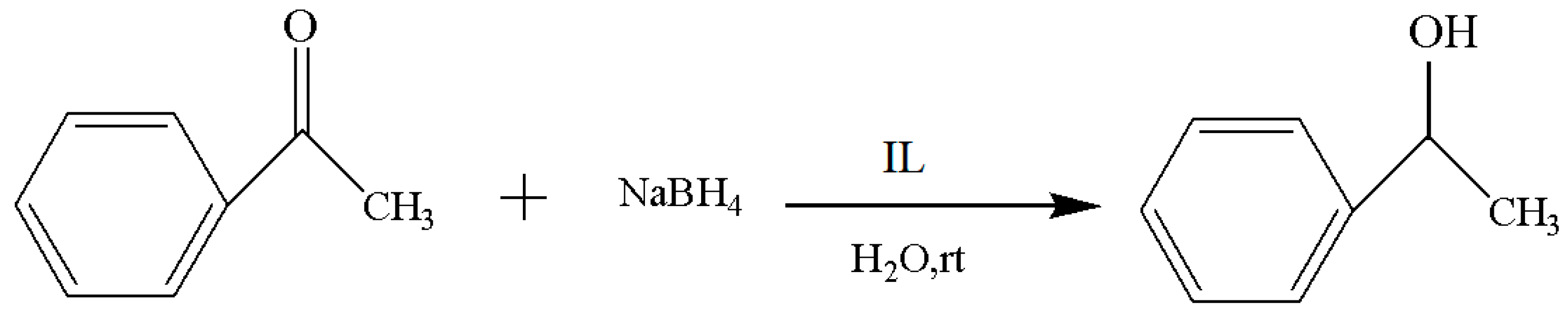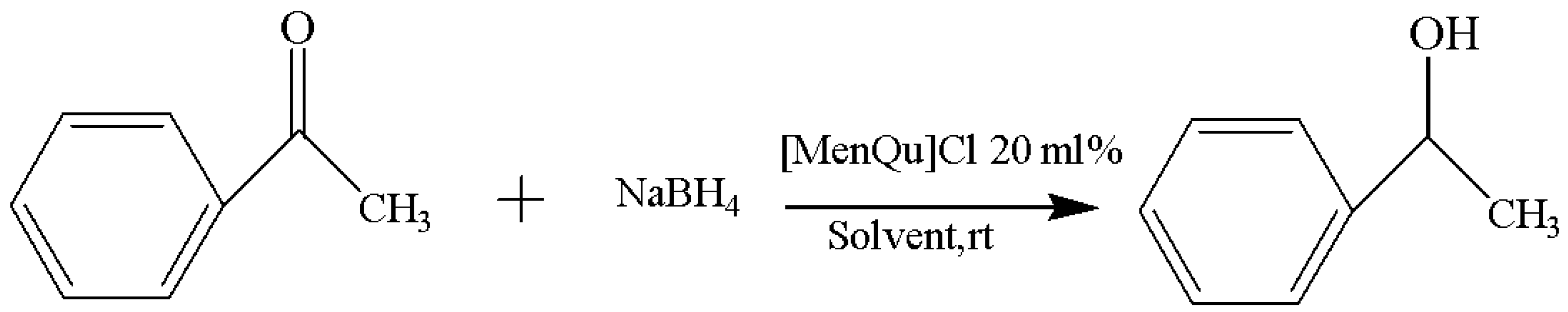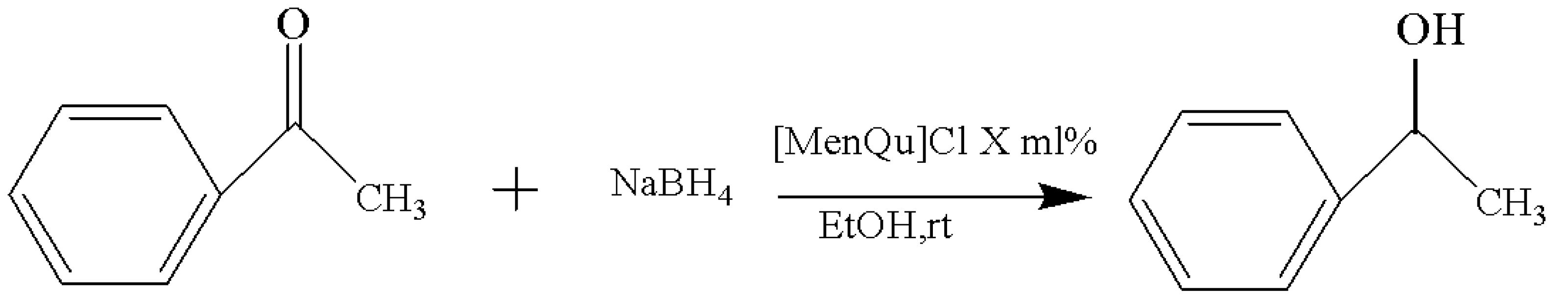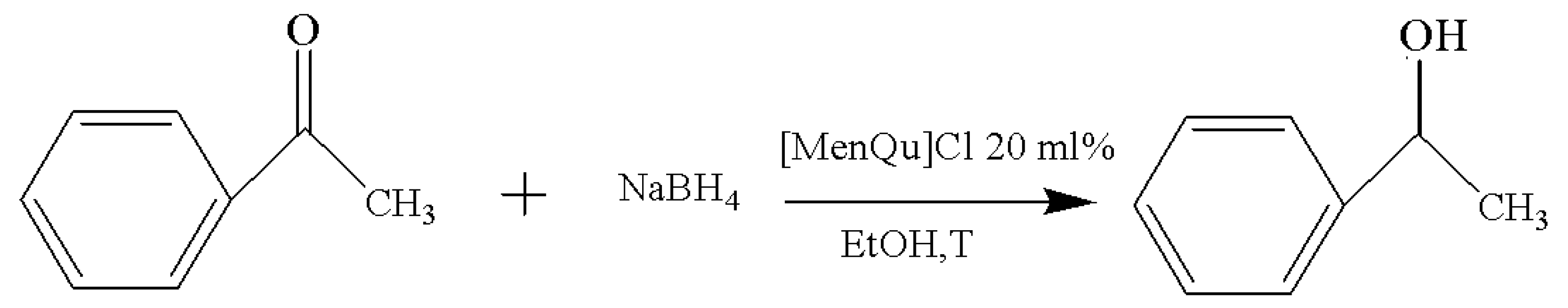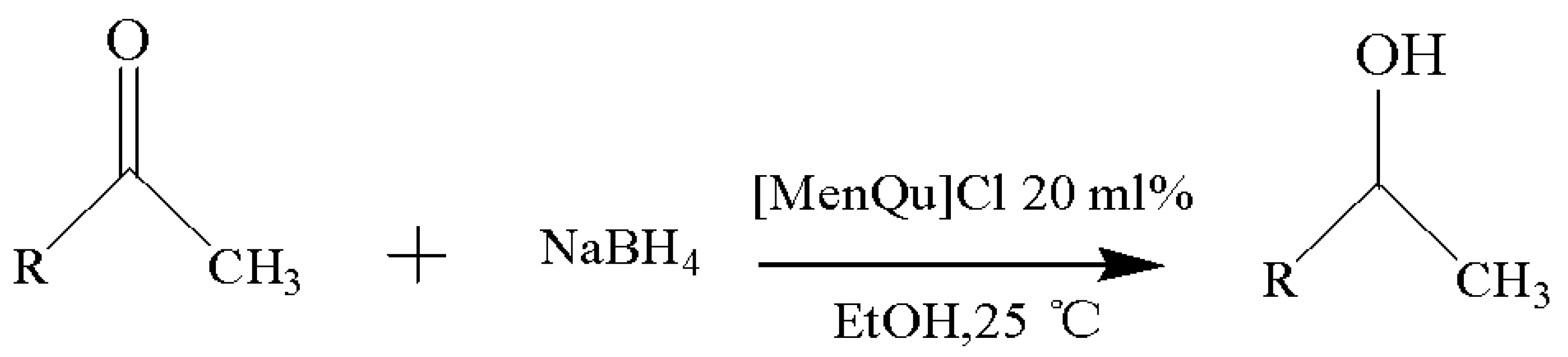Abstract
As a new sustainable catalyst and green solvent, ionic liquid has important applications in catalytic reduction. In this study, four quinuclidinol-based quaternary ammonium ionic liquids (ILs)were at first prepared using simple procedures, and then they were used as catalyst for acetophenone reduction. The main catalytic conditions were studied and screened through a comparison among the reaction results. It showed that the highest yield of 1-phenylethanol was 88.6% when [MenQu]Cl was used as a catalyst; the ideal solvent was ethanol; the amount of catalyst was 20 mol%, and the reaction temperature was 25 °C. More substrates were employed to validate the universality of the developed method using such an IL. Moreover, potential reaction mechanisms and an IL recovery method were also suggested, which laid the foundation for its further applications in the field of sustainable chemistry and cleaner industrial production.
1. Introduction
A cleaner and friendly technological route is the focus of both academic and industrial circles. As one of the new sustainable means to achieve an intensive development mode of economy and society, the use of ionic liquid (IL) is becoming more and more attractive. Ionic liquid is a kind of room-temperature-molten salt composed of organic cations and inorganic anions. It has many characteristics, such as its low vapor pressure, non-volatility, easy recovery, good stability, and so on. It has gradually become a good substitute for traditional organic solvents [1]. The application of this green solvent for catalytic reduction is an important sustainable way to solve the shortcomings of the existing reaction system. It has been widely used in organic synthesis because of advantages such as its high activity, strong catalytic effect, broad solubility, friendliness, adjustable function, flexible applied forms, low production cost, etc. Ionic liquids can be used as catalysts for olefine reduction, nitro reduction, carbonyl reduction, and other reactions and have a great application prospect in many reaction systems [2,3,4], especially for those used for the preparation of products with great value in the medical and healthcare fields.
Acetophenone, also known as acetylbenzene, is the simplest aromatic ketone, and its aromatic nucleus is directly connected to a carbonyl group. Acetophenone exists in the essential oils of some plants in a free state and has an aroma like hawthorn, so it can be used to prepare spices and as a raw material for pharmaceutical and other organic synthesis. Acetophenone can be reduced to form 1-phenylethanol (also known as α-phenylethylalcohol). This kind of product is a colorless liquid with a light gardenia flavor, which also has a wide range of applications in the pharmaceutical field. Benzeneethanol can be used as an analgesic, antibacterial, antiviral, and anti-tumor agent, among other things, and it can also be used as an intermediate in the preparation of some drugs, such as phenylethanolamine, phenylethanolic acid, etc. Traditional acetophenone reduction systems have shortcomings such as a low catalytic efficiency, high reagent costs, unfriendly conditions, and poor sustainability, which urgently need improvement [5].
At present, the applied ILs for reduction reactions include [Bmim][Br], [Bmim][BF4], [Bmim][AlmCln], [DMEA][Lac], [HBth]HSO4, etc. These green sustainable solvents have been found to contribute to the formation of intermediates, reducing the activation energy and enhancing system miscibility. Overall, the current IL types are very limited, mainly consisting of the imidazolium type, and there is still room for further improvement in the yield of reduction products (usually below 80%). For instance, electroreduction with sodium borohydrate occurs in ionic liquids [Bmim][Br]/water mixtures. When a certain amount of ionic liquid is added to pure water as a reaction solvent, the substrate and sodium borohydrate dissolved in the water are changed from a two-phase system to a homogeneous system, with a final product yield of 75% [6]. In order to expand the types of IL used for reduction and explore potential reaction mechanisms, this study prepared a unique type of IL using quinolinol and menthol as the main raw materials, exploring its potential application possibilities in the catalytic field.
2. Experiment
2.1. Reagents and Materials
Acetophenone and isopropanol were provided byKelong Chemical Plant (Chengdu, China). R-3-quinuclidinol, menthol, chloroacetic acid, o-hydroxyacetophenone, o-methylacetophenone, p-methylacetophenone, p-nitroacetophenone, parabromoacetophenone, potassium hexafluorophosphate, potassium trifluoromethanesulfonate, sodium tetrafluoroborate, and p-bromophenone were purchased from Adamas Reagent Company (Shanghai, China). Silica thin-layer plates were sourced from Ocean Chemicals (Qingdao, China). N-hexane was purchased from Thermo Fisher Scientific (Waltham, MA, USA). Except for the chromatographic-grade isopropanol and n-hexane used for a quantitative analysis, all the reagents and solvents were of an analytical pure grade and were used without further purification if not stated otherwise.
2.2. Instruments
The quantitative determination of 1-phenylethanol was performed usingLC3000 high-performance liquid chromatography (Innovation Tongheng Technology Co., Ltd., Beijing, China) equipped with a Welchrom-C18 chromatographic column (4.6 mm × 250 mm, 5 μm) with methanol-water (60:40, v/v) and detected at 254 nm. When the enantiomers of the 1-phenylethanol were analyzed, the Chiralcel OD-H column (250 × 4.6 mm, 5 μm; DAICEL Inc., Tokyo, Japan) was used with an n-Hexane and iso-propanol mixture (95:5, v/v) in an isocratic mode at 0.9 mL/min [7].
2.3. Synthesis of Quinuclidinol-Based Quaternyl Ammonium Ionic Liquids
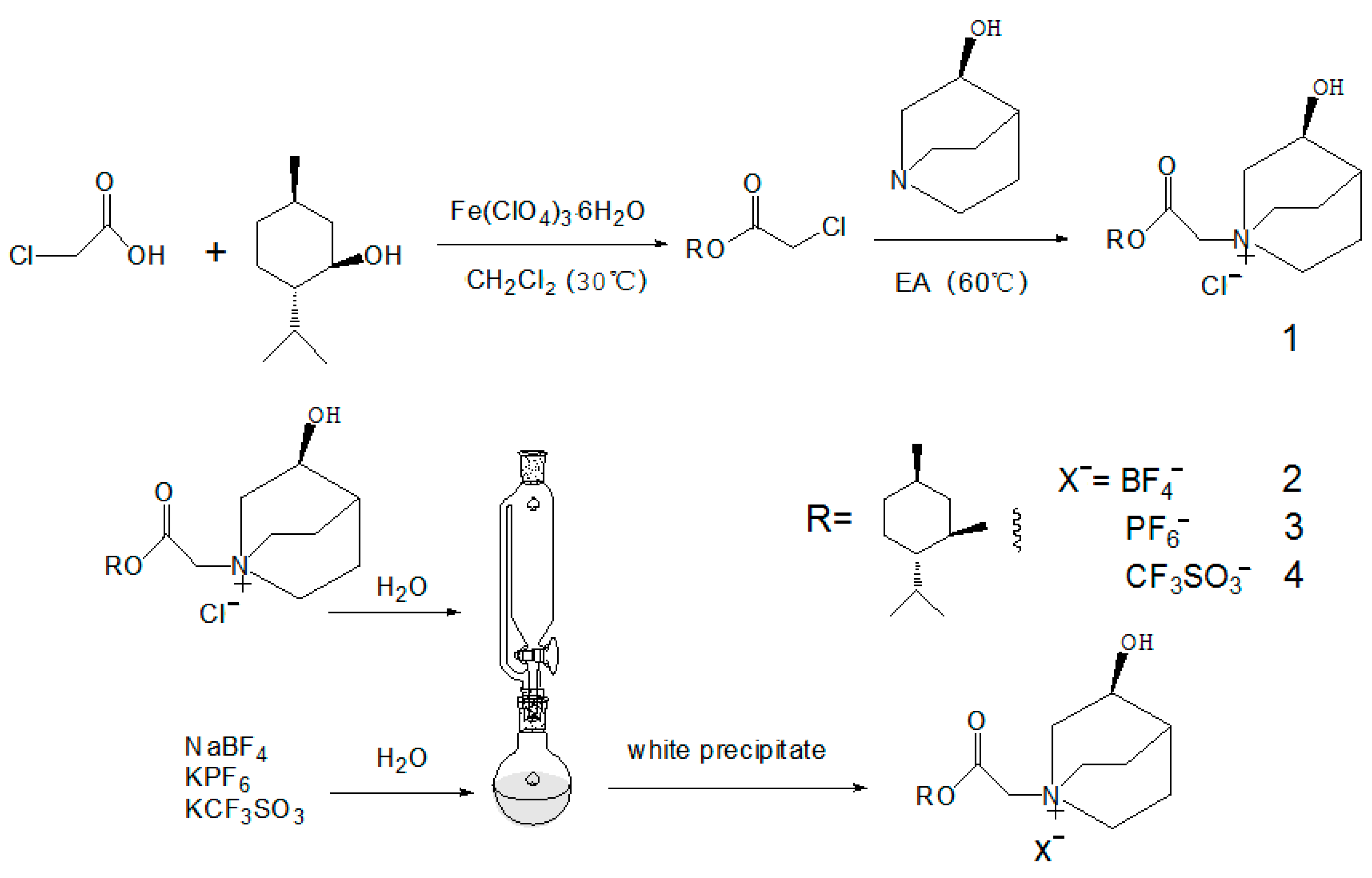
Taking the IL of [MenQu]Clas an example (see Figure 1), firstly, menthol and chloroacetic acid were combined to obtain the product of menthol chloroacetate rapidly. Secondly, 30 mL ethyl acetate was poured into a single neck bottom flask of 100 mL; then 5 mmol (0.635 g) of R-3-quinuclidinol was added, and ultrasonic oscillation was applied to assist its dissolution. After thorough dissolution, the round bottom bottle was put into an oil bath at 40 °C for heat preservation, and magnetic stirring was turned on. In addition, 5 mmol of menthol chloroacetate(1.164 g) was dissolved in 10 mL of ethyl acetate and added in the reactive system using the drop funnel with a speed of one to two drops per second. After adding the menthol chloroacetate solution, the drop funnel was replaced with a spherical condensate tube, and the condensate water was turned on; meanwhile, the oil bath’s temperature was raised to 60 °C to continue the reaction. During the reaction, white solids continued to precipitate. After 5 h of reaction, the heating and stirring power was turned off. After the reactant was slightly cold, a great amount of white precipitates were obtained. After filtration, the filter cake was washed with an appropriate volume of ethyl acetate for three times to remove unreacted components and other impurities. After drying, the white solid was placed in the air-dryer (50 °C) for 24 h and then transferred to the vacuum-dryer for preservation. Three other ionic liquids with the same cation could be obtained via an anion exchange with corresponding salts, and then Cl− was changed to be BF4−, PF6−, and CF3SO3−. All the IL products were checked and confirmed using a spectral analysis.
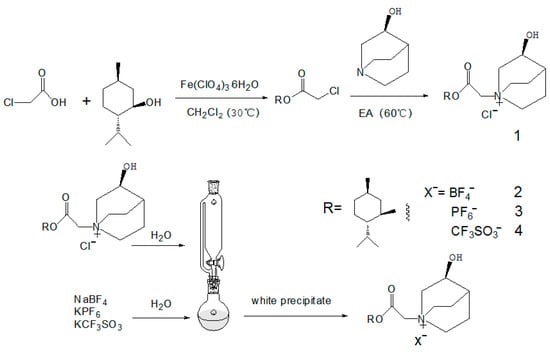
Figure 1.
Schematic diagram of the experimental process of quinuclidinol-based quaternyl ammonium ILs (1: [MenQu]Cl; 2: [MenQu]BF4; 3: [MenQu]PF6; and 4: [MenQu]CF3SO3).
2.4. Reduction of Acetophenone
At room temperature, 5 mL of solvent (water/acetone/methanol, etc.) was added to the 25 mL round bottom flask; then, 1 mmol (0.1202 g) of acetophenone and 20 mol% of quinuclidinol-based quaternyl ammonium IL were also added to the flask with thorough mixing. Then, 0.0567 g (1.5 mmol) of sodium tetrafluoroborate was slowly added to the reactive system under continuous magnetic stirring. The reaction process was detected using thin-layer chromatography with chloroform-methanol as the developing reagent, and the position and size of the reactants and product spots were observed under a UV lamp with a wavelength of 254 nm. When the spots of the reactants completely disappeared, 5 mL of saturated ammonium chloride solution was added in the reactive system to quench the reduction reaction; then, 8 mL of ethyl acetate was applied to extract the product of 1-phenylethanol from the reaction solution three times, which could be obtained with liquid–liquid separation and concentration under vacuum. The yield was calculated using the results through liquid chromatography.
3. Results and Discussion
3.1. Investigation on Reaction Conditions
3.1.1. Effect of the IL Anions on the Reaction Results
The applied frequency of the Cl−, BF4−, PF6−, and CF3SO3−of the IL is high for the catalysis in the current study, and the performance difference among them was investigated first. The experimental results are included in Table 1, and the reaction route is shown in Figure 2. After comparing the yield and reaction time of the reduction process, it was found that the IL with the best performance was [MenQu]Cl, and the yield was 77.7% (that of the other three was below 70%); moreover, the reaction duration was less than 3 h. The reason is that the polarity of ionic liquids depends on their anions when their cation remains unchanged; meanwhile, the reactive system is more inclined to form homogenization in the IL with Cl− than in the ILs with BF4−, PF6−, and CF3SO3−, so their reaction efficiency differed [8,9].

Table 1.
Yields of catalytic reactions with four ILs with different anions.
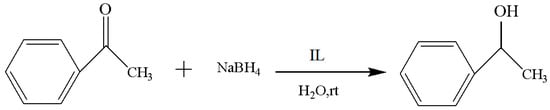
Figure 2.
Reaction scheme for the investigation of IL anions.
3.1.2. Effect of Different Solvents on the Reaction Results
The experimental results of solvent replacement are summarized in Table 2, and the reaction route is shown in Figure 3. After screening the solvents, the best candidate is found to be EtOH, which can result in the highest yield (88.6%) within the shortest duration (1 h). This may be related to the viscosity, polarity, and solubility of these solvents, and the reaction with the solvent system with a good miscibility with acetophenone is faster [10]. To make the reaction more efficient, ethanol was selected as the ideal solvent for the reaction in subsequent experiments.

Table 2.
Results of catalytic reactions in different solvents.
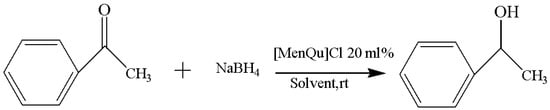
Figure 3.
Reaction scheme for the investigation on different solvents.
3.1.3. Effect of IL Dosage on the Reaction Results
Through the screening experiment of catalyst dosage, it is found that the amount of IL catalyst has little effect on the experimental results; the specific results are included in Table 3, and the reaction route is shown in Figure 4. This suggests that a small amount of IL can achieve the desired catalytic effect and that its activity is very high, while a large amount of IL can actually cause an increase in system viscosity, as well as a difficult recovery and unnecessary waste; at the same time, the probability of side reactions may also increase as a result [11]. Finally, the 20 mol% [MenQu]Cl was selected to explore the subsequent reaction conditions.

Table 3.
Effect of IL dosage on yield of reaction results.
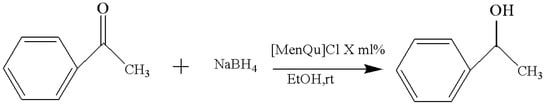
Figure 4.
Reaction scheme for the investigation on IL dosage.
3.1.4. Effect of Temperature on the Reaction
Based on the reaction route in Figure 5, the data in Table 4 show that the change of temperature has a less obvious effect on the yield of the reaction. Considering that the freezing point of ethanol is much lower than 0 °C, here, we have investigated the reaction results below the freezing point. It is found that mild conditions under room temperature can achieve the satisfied results. However, with the decrease in temperature, the reaction time will be prolonged; meanwhile, it still maintains a yield near 70% at −20 °C. Previous studies also indicate that the temperature may affect the reaction efficiency without noticeably changing the reaction yield, but it can have an impact on enantiomers [12,13]. According to the experimental screening results, the subsequent reaction at a room temperature of 25 °C was selected.
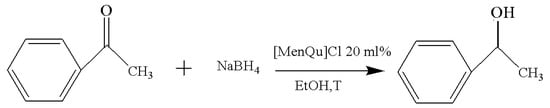
Figure 5.
Reaction scheme for the investigation on temperature.

Table 4.
Effect of temperature on reaction results.
3.2. Expansion of Reaction Substrate
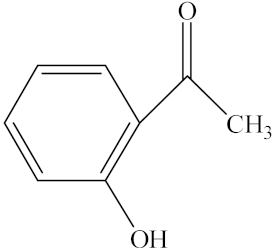
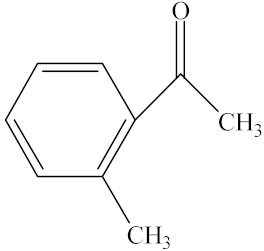
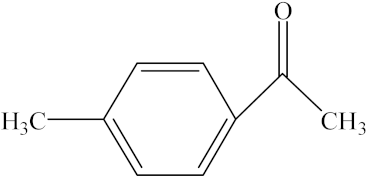
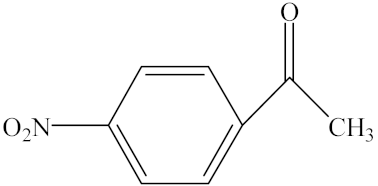
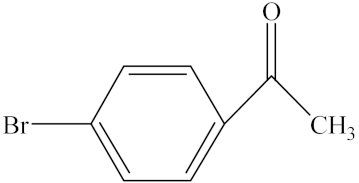
With the reaction route in Figure 6, it is found from the results in Table 5 that the developed IL-based catalytic method is applicable to other substrates with similar structures of acetophenone. Here, o-hydroxyacetophenone, o-methylacetophenone, p-methylacetophenone, p-nitroacetophenone, and parabromoacetophenone were selected to make comparison with acetophenone. It can be found that different R groups have some influence on the reaction carbonyl group and that the reaction efficiency of the electron-absorbing group is higher [12]. Therefore, the best catalytic result is still obtained with the [MenQu]Cl-promoted reduction of p-nitroacetophenone within 0.5 h, and the reaction speed is high.
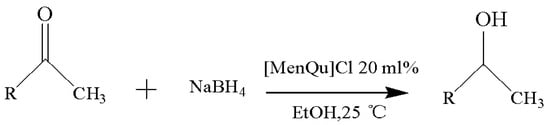
Figure 6.
Reaction scheme for the investigation on different substrates.

Table 5.
Reaction results of different substrates.
3.3. Potential Reaction Mechanism
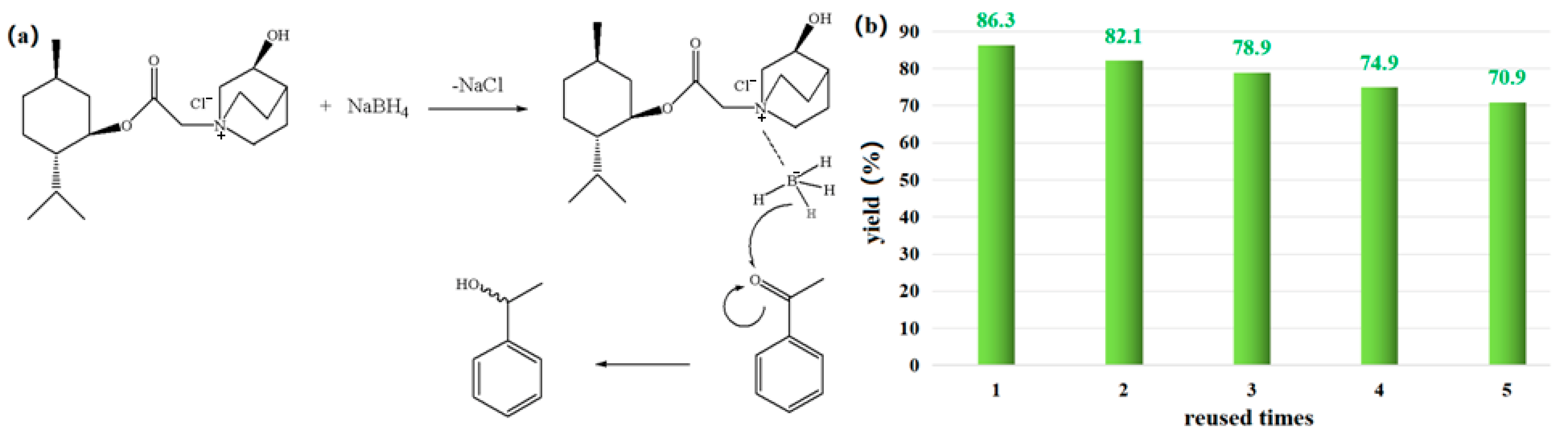
Based on the above results and the existing literature [5,6], the following potential mechanism is suggested. As shown in Figure 7a, sodium borohydride first forms a reductive borane complex with the ionic liquid, and then a hydrogen of borane is removed, attacking the carbonyl’s carbon-positive ion, finally forming a new chiral center. If the system where the N+ center is located has a stabilizing effect on the carbonyl group of acetophenone, the transition state is easier to form and more stable. Furthermore, through the steric hindrance in the menthol plane and the steric hindrance outside the ring of quinuclidinol, the spatial steric hindrance of menthol is promoted; the anions of BH4−from the back of the bridge ring approach the center of N+, so acetophenone is close to the anions of BH4−from the back. Therefore, the main product should be 1-phenylethanol with α-OH, which has been confirmed with the enantioseparation results using achiral chromatographic column under the analytical conditions in Section 2.2.
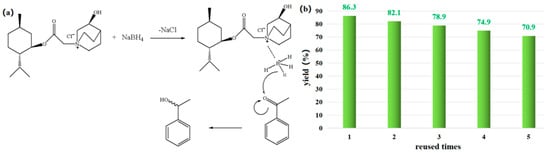
Figure 7.
(a) Possible reduction mechanisms catalyzed via IL and (b) yield in the IL reused process.
3.4. Recovery and Reuse of IL
As a sustainable solvent, a good recyclability is required for [MenQu]Cl. After the reaction was completed, ethyl acetate was used to extract the product, and then isochoric n-butanol was further applied to extract and recover the ionic liquid from the residual solution two times. After back-washing with redistilled water, the n-butanol extract was concentrated and dried under vacuum and then used for the next parallel reuse experiment. The results of five recycling experiments are shown in Figure 7b (when the reused time = 0, yield = 88.6%), indicating that the above recovery method can maintain an acceptable IL catalytic activity over a certain number of cycles. But, when the color of the recovered [MenQu]Cl was found to have significantly deepened, a more complex regeneration way was needed, and the experimental results suggested that the D101 macroporous resin can achieve a satisfactory performance with 95% of ethanol as the eluent reagent.
4. Conclusions
The newly synthesized quinuclidinol-based quaternary ammonium ionic liquids were applied to the hydrogenation and reduction of chiral ketones. The optimum catalytic conditions were determined by screening the catalyst type, reaction solvent, catalyst dosage, reaction temperature, and so on. Through the investigation on their effects, the results indicate that the catalytic activity of different ILs from high to low follows the order of [MenQu]Cl > [MenQu]BF4 > [MenQu]PF6 > [MenQu]CF3SO3. The reaction can be completed under mild conditions around room temperature, and a low IL dosage is enough for its effective effect. The most effective catalytic conditions were as follows: [MenQu]Cl as a catalyst, ethanol as a solvent, a catalyst dosage of 20 mol%, and a reaction temperature of 25 °C. The yield of 1-phenylethanol was 88.6%. It was found in the substrate expansion experiments that similar substrates can be also suitable for such an IL catalyst, which means that it has good universality. On the basis of the above results and current studies, possible experimental mechanisms were suggested, which provided a meaningful reference for the subsequent structure optimization of the catalyst. Finally, the IL was well-reused through simple recovery procedures, and it is necessary for sustainable chemistry and cleaner production processes.
Author Contributions
Conceptualization, G.Z. and R.L.; methodology, G.Z.; software, H.C.; validation, Y.C.; formal analysis, R.L.; data curation, H.C.; writing—original draft preparation, G.Z.; writing—review and editing, S.Y.; visualization, Y.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Sichuan Science and Technology Program (No. 2021YFG0276).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
All the authors’ affiliations provided the convenience for the related studies, respectively. A special thanks goes to the Engineering Experimental Teaching Center, in the School of Chemical Engineering at Sichuan University, for related measurements and characterizations, including FT-IR and NMR, etc.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pendleton, J.N.; Gilmore, B.F. The antimicrobial potential of ionic liquids: A source of chemical diversity for infection and biofilm control. Inter. J. Antimicrob. Agents 2015, 46, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Han, B.; Liu, Z. Ionic-liquid-catalyzed approaches under metal-free conditions. Acc. Chem. Res. 2021, 54, 3172–3190. [Google Scholar] [CrossRef] [PubMed]
- Chinnappan, A.; Tamboli, A.H.; Chung, W.J.; Kim, H. Green synthesis, characterization and catalytic efficiency of hypercross-linked porous polymeric ionic liquid networks towards 4-nitrophenol reduction. Chem. Eng. J. 2016, 285, 554–561. [Google Scholar] [CrossRef]
- Javle, B.R.; Kinage, A.K. Chiral amino-acid-amide based ionic liquids as a stereoselective organocatalyst in asymmetric transfer hydrogenation of acetophenone at room-temperature. Chemistryselect 2018, 3, 2365–6549. [Google Scholar] [CrossRef]
- Singh, S.K.; Savoy, A.W. Ionic liquids synthesis and applications: An overview. J. Mol. Liq. 2020, 297, 112038. [Google Scholar] [CrossRef]
- Su, Z.; Zhang, L.; Zhang, Y.P. Ionic liquid/water mixed solvent promotes the reduction of aldosterone. J. Lanzhou Univ. Technol. 2008, 34, 74–76. [Google Scholar]
- Özlem, A.; Emine, B.; Ülkü, M. Determination of effective diffusion coefficient of acetophenone in κ-carrageenan and asymmetric bioreduction in packed bed reactor. J. Mol. Catal. B Enzym. 2011, 72, 46–52. [Google Scholar]
- Yu, B.; Zhou, F.; Liu, G.; Liang, Y.; Huck, W.T.S.; Liu, W. The electrolyte switchable solubility of multi-walled carbon nanotube/ionic liquid (MWCNT/IL) hybrids. Chem. Commun. 2006, 22, 2356–2358. [Google Scholar] [CrossRef]
- Ogoshi, T.; Onodera, T.; Yamagishi, T.-A.; Nakamoto, Y. Green polymerization of phenol in ionic liquids. Macromolecules 2008, 41, 8533–8536. [Google Scholar] [CrossRef]
- Bertero, N.M.; Trasarti, A.F.; Apesteguía, C.R.; Marchi, A.J. Solvent effect in the liquid-phase hydrogenation of acetophenone over Ni/SiO2: A comprehensive study of the phenomenon. Appl. Catal. A Gen. 2011, 394, 228–238. [Google Scholar] [CrossRef]
- Akopyan, A.V.; Eseva, E.; Polikarpova, P.; Kedalo, A.; Vutolkina, A.; Glotov, A.P. Deep Oxidative desulfurization of fuels in the presence of brönsted acidic polyoxometalate-based ionic liquids. Molecules 2020, 25, 536. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wei, T.; Zhang, Q. Effect of Temperature on the Enantioselectivity in the Oxazaborolidine-Catalyzed Asymmetric Reduction of Ketones. Noncatalytic Borane Reduction, a Non-neglectable Factor in the Reduction System. J. Org. Chem. 2003, 68, 10146–10151. [Google Scholar] [CrossRef]
- Sun, W.; Xia, C.G.; Zhao, P.Q. Chiral salen-Co(II) complex catalyzed asymmetric reduction of acetophenone with sodium borohydride. Acta Chim. Sin. 2001, 59, 976–978. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).