Abstract
Laser-induced breakdown spectroscopy (LIBS) was explored to assess the element constituents (Ca, Mg and N) in the skin, pulp, and seed of two Vitis vinifera cultivars—a white (Loureiro) and a red (Vinhão) cultivar. This study compares the two grape cultivars chosen and the characterisation of Ca, Mg and N in the skin, pulp and seed on three dates after veraison. Significant differences (p < 0.05) were found in the Ca, Mg and N in the skin, pulp and seed of both grape cultivars during the three assessment dates considered. The results of this study could provide insights into the element composition of grapes, offering a fast, accurate, and cost-effective alternative to traditional element quantification methods.
1. Introduction
The composition of grapes influences the taste, nutritional value, and overall quality parameters of the grapes [1]. Calcium (Ca), magnesium (Mg), and nitrogen (N) are essential macronutrients that play pivotal roles in the growth, development, and metabolic processes of grapevines [2]. Ca is necessary for membrane stability and empowers disease resistance and metabolic pathways [2]. Mg drives photosynthesis and enzyme activation, nurturing vine health [1]. N shapes vital proteins, optimising growth, development, and canopy vitality [2]. Consequently, the presence and distribution of these constituents in grape tissues are critical factors affecting the resulting yield and fruit quality [3].
Conventional chemical methods have been traditionally employed to characterise element content in grape tissues. However, these methods bear limitations that hinder their utilisation for routine analysis in the viticultural industry. For instance, acid digestion methods coupled with atomic absorption spectrophotometry (AAS) or inductively coupled plasma–optical emission spectrometry (ICP-OES) require considerable sample preparation, leading to time-consuming and labour-intensive procedures [4]. Furthermore, requiring highly specialised equipment, reagents, and skilled operators renders these techniques expensive, posing challenges for regular analyses in large-scale vineyards.
Alternative non-destructive methods for quantifying element constituents have been developed. X-ray fluorescence has found application in characterising element composition in biological samples [5]. However, these methods may be limited to quantifying elements with atomic numbers (Z) lower than 12 [5]. Additionally, it is crucial to consider potential radiation effects stemming from the equipment during the analysis process.
To bypass the shortcomings of traditional elemental quantification techniques, Laser-Induced Breakdown Spectroscopy (LIBS) has emerged as a viable alternative method for elemental analysis in diverse fields, including agriculture and food science [3,6]. LIBS is a rapid, destructive analytical technique that utilises a laser pulse to ablate and excite the object under analysis (e.g., grape tissue), generating a plasma plume. Subsequently, the emitted optical radiation from the plasma is collected and analysed to determine the sample’s elemental composition, which is a limitation of LIBS since it primarily identifies the accumulation of elements like Ca but does not provide information about their molecular structure [4]. Due to its inherent advantages of minimal sample preparation, high spatial resolution, and reduced operational costs, LIBS has gained popularity as a promising method for elemental tissue analysis in grapes [7].
Through the implementation of LIBS, this study aims to characterise Ca, Mg and N in the skin, pulp and seed of two grape cultivars along the maturation.
2. Materials and Methods
2.1. Test Site
The study was carried out in the Região dos Vinhos Verdes, situated in the northwest region of Portugal, specifically at the Campus Agrário de Vairão (41°19′31.91″ N; 8°40′27.45″ W) in Vila do Conde. Grape sampling occurred in the year 2020 after veraison (August 14th), containing two grape cultivars: Loureiro (Vitis international variety catalogue (VIVC) 25085), a white grape cultivar, and Vinhão (VIVC 13100), a red grape cultivar. The sampling was conducted on three different dates for both Loureiro and Vinhão cultivars: September 30th (S1), October 12th (S2), and October 14th (S3). A total of 39 grapes were gathered for the Loureiro cultivar (S1: n = 20, S2: n = 9, S3: n = 10), while 29 grapes were collected for the Vinhão cultivar (S1: n = 16, S2: n = 8, S3: n = 5).
2.2. Characterisation of the Grapes
Grape tissues were meticulously separated into skin, pulp, and seed sections using a scalpel. The tissues were weighed and subsequently placed in an oven at 45 °C for four days to dry. After this period, the dried tissues were re-weighed, allowing for the determination of water content (%) through the weight difference.
A grinder was used to individually macerate the sample’s dried tissues. A palette with a volume of approximately 0.098 cm3 from these dried tissues was assembled for subsequent analysis using LIBS.
2.3. LIBS Instrumentation
A conventional LIBS experimental configuration was employed in this study. A ytterbium-doped fibre laser (MWTechnologies, model PFL-1064-FL-10kW, M2 = 1.4) generated pulses at a 1064.8 nm wavelength, with a diameter of 0.51 mm. The laser could operate in single-pulse mode or with a repetition rate of up to 250 kHz. Pulse durations ranged from 5 to 305 ns, allowing fine control of their shape at 5 ns intervals.
The pulses were expanded using a beam expander (×12) and then focused onto the sample surface using a plano-convex lens with a focal length of 100 mm. The resultant spectra were captured using a two-lens optical system, routed through a 200 µm optical fibre, and directed to a spectrometer (Avantes, model AvaSpec-ULS2048CL-EVO, Apeldoorn, The Netherlands) covering the range of 482–615 nm with a resolution of less than 0.1 nm.
Single laser shots were applied to the sample for each pulse width. The acquired spectra had an integration time of approximately 30 µs, encompassing the entire duration of the plasma emission (non-gated). During data analysis, the set of 10 spectra for each pulse width was averaged, and background and baseline noise was eliminated using an asymmetric least-squares method [8,9].
2.4. Element Identification
This study employed a qualitative approach to distinguish the presence of Ca, Mg, and N within the skin, pulp, and seeds. The central objective of LIBS is to deduce the chemical composition of samples by analysing their spectra. Each chemical element emits light at distinct wavelengths, resulting in well-defined lines within the spectrum. However, scenarios might arise in which emission wavelengths of different elements overlap.
Two reference databases—OSCAR [10] and NIST database [11]—were evaluated in order to understand their appropriateness to the samples’ spectra.
2.5. Statistical Analysis
An ANOVA associated with a Duncan post hoc test (when needed) was performed to assess the difference in the water content in both grape cultivars in the different tissues along the three assessment dates. This was also conducted to see if there were significant statistical differences among the characterisation of Ca, Mg and N in the skin, pulp and seeds of both grape cultivars along the three assessment dates. The non-significant and significant (p < 0.05) were considered in this study.
3. Results
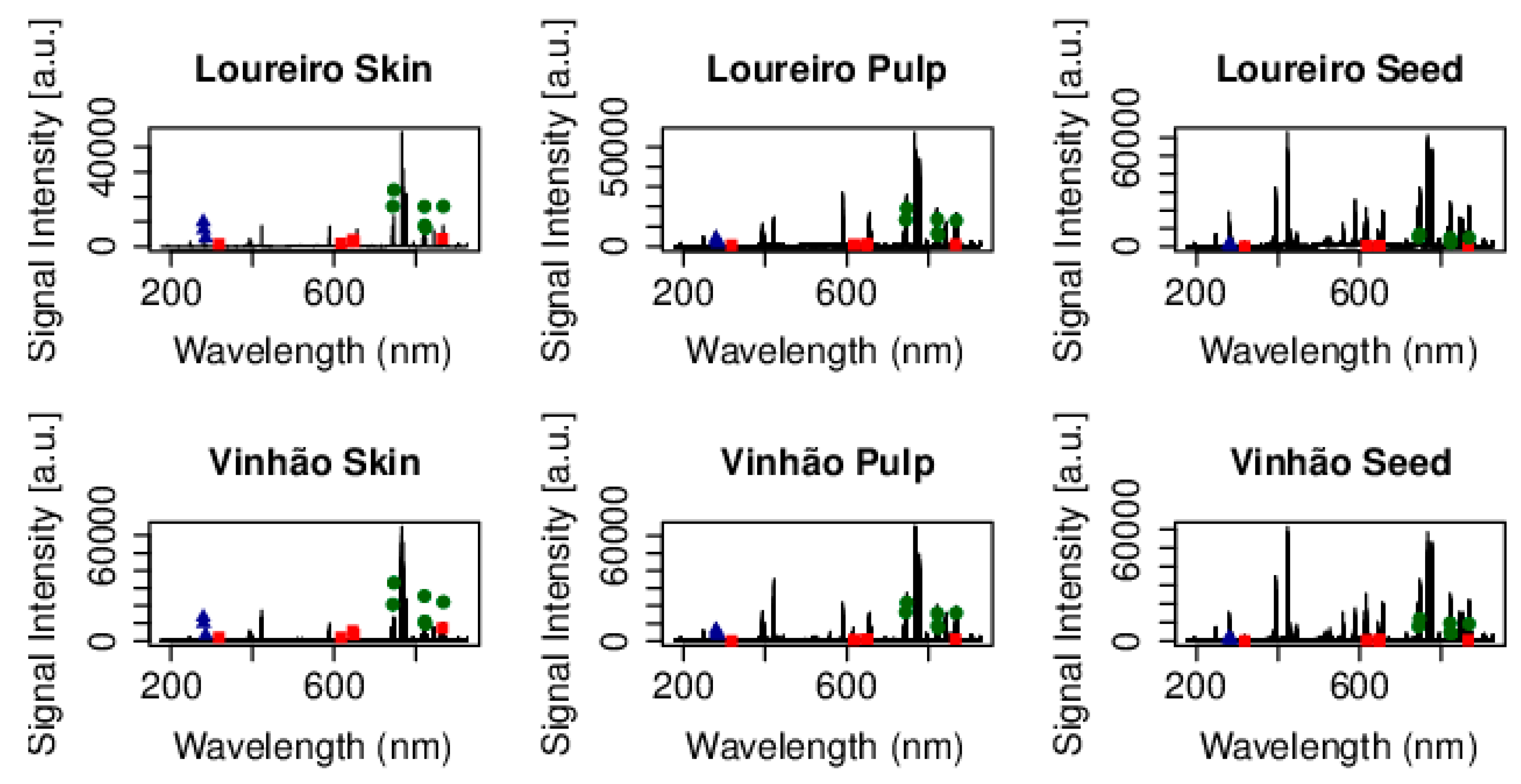
Figure 1 shows the average signal intensity for the two grape cultivars regarding skin, pulp, and seed in S1. This figure includes the corresponding wavelengths representing each element. Ca displayed five discernible peaks: 317.936 nm, 617.128 nm, 646.273 nm, 649.378 nm, and 866.097 nm. Mg showcased three peaks: 279.535 nm, 280.24 nm, and 285.18 nm; whereas N revealed six distinct peaks: 744.2 nm, 746.753 nm, 821.533 nm, 822.248 nm, 824.184 nm, and 867.938 nm.
Figure 1.
The mean signal intensity of the Ca (!), Mg (7) and N (,) for the skin, pulp and seed of the Loureiro (n = 20) and Vinhão (n = 16) cultivars in S1.
Table 1 presents statistical disparities in Ca, Mg, and N concentrations across the two grape cultivars’ skin, pulp, and seed tissues during the three assessment dates. Regarding Ca concentrations within these tissues, the Loureiro cultivar displays significant variations across all three assessment dates for all tissues. To the contrary, the Vinhão cultivar exhibits divergence only in the pulp tissue. A comparative analysis of the two cultivars reveals uniform Ca concentrations in the skin and consistent concentrations in the seeds during S3.

Table 1.
Average LIBS spectra intensity peaks of Ca, Mg and N and water content (% w/w) for each tissue in the three dates of assessment (S1, S2 and S3) for the Loureiro and Vinhão cultivars.
Regarding Mg content, the Loureiro cultivar demonstrates variations across the three assessment dates for both skin and pulp tissues. Conversely, the Vinhão cultivar remains consistent in Mg concentrations across the internal tissues over the three assessment dates. A cross-cultivar examination reveals that the skin tissue of the Loureiro cultivar during S3 exhibits significant differences in Mg content, along with the pulp and seed tissues in S1 and S2.
In the context of N content analysis, the Loureiro cultivar showcases a lack of significant differences in skin tissue across the three assessment dates. In contrast, the Vinhão cultivar presents statistical distinctions solely in the seed tissue. An evaluation of N concentrations between the two cultivars shows significant disparities in pulp tissue for both grape cultivars throughout the three assessment dates and in the seed tissue during S3.
The examination of water content reveals significant changes in the skin and pulp tissues of the Loureiro cultivar, while the seed tissue remains unaffected. Conversely, the Vinhão cultivar’s water content experiences fluctuations across all three grape cultivars. Significant deviations in water content for both cultivars manifest in the skin tissue in S3 and in the pulp and seed tissues in S1 and S3. Also, the water content may have affected the concentration of the macro elements studied more in the Loureiro cultivar than in the Vinhão cultivar.
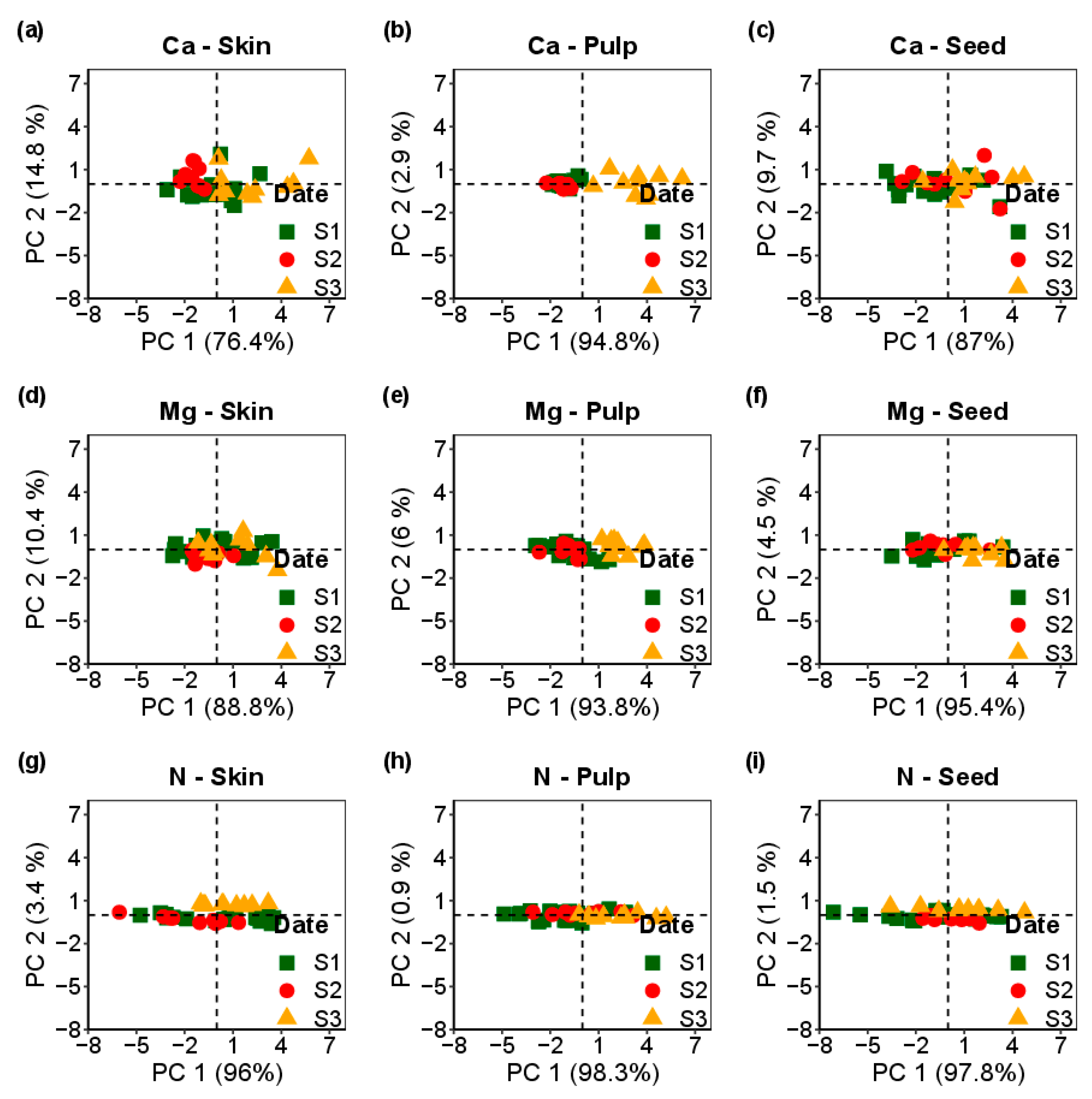
Comparing the three post-veraison dates, Figure 2 demonstrates the dynamic changes of Ca, Mg, and N in the Loureiro cultivar regarding the spectral data of the skin, pulp, and seed. These dynamics are observed across the assessment dates. The results underscore principal component 1 (PC1) and PC2 representability, collectively accounting for over 90% of the variance. Furthermore, the outcomes follow Table 1, suggesting differences in Ca in the skin, pulp and seed for the three assessment dates, differences in Mg content in the skin and pulp, and differences in the pulp and seed regarding N.
Figure 2.
Principal component analysis of the skin, pulp and seed of the Loureiro cultivar according to the three dates of assessment: S1, S2 and S3.
4. Discussion
The application of LIBS within viticulture presents a promising pathway as an alternative to conventional wet-lab techniques, which are known for their resource-intensive nature. This study embarks on an initial exploration into the potential utilisation of LIBS across diverse grape tissues. By employing a qualitative framework, the investigation is centred on identifying essential elements, namely Ca, Mg, and N, within distinct segments —skin, pulp, and seed—of two grape cultivars.
Variations in water content (Table 1) influence the elemental constitution of tissues in grape cultivars [1], especially in white cultivars, as demonstrated in this study. The outcomes articulated within this study underscore pronounced disparities in the concentrations of Ca, Mg and N across different grape cultivars in the three assessment dates (Table 1). Furthermore, the existing literature attests to a tendency for Ca and Mg to accumulate during the maturation of grapes [1,3], thereby illustrating the temporal pattern in element composition exhibited among these distinct cultivars. Similarly, the difference in N content in all tissues along the maturation of both grape cultivars (Table 1, Figure 2) represents a vital approach to plant metabolism [2].
This study introduces an exploratory application of LIBS for elemental analysis in grapes. The potential exists for enhanced refinement and validation of this technique, which could yield cost-effective and expeditious alternatives to established methods for evaluating element content in grape tissues. The limited biological material utilised in this investigation could pose challenges for wet-lab procedures, necessitating adaptations that entail substantial costs and time commitments. Nonetheless, with comprehensive validation, the deployment of LIBS holds promise for on-field elemental composition characterisation [6], potentially spurring further metabolomics research endeavours.
5. Conclusions
This work characterised the Ca, Mg and N of grapes in the skin, pulp and seed using LIBS. It demonstrated differences in the elemental composition of the grape cultivars studied along the maturation.
LIBS presents great potential as a cheap and faster alternative to determining the elemental composition of grapes. The validation of LIBS could bring more studies related to plant physiology and promote more studies in precision viticulture. The wet-lab proof of the results of LIBS could also extend the utilisation of LIBS for the determination of other macro and micro elements in grapes and other fruits.
Author Contributions
Conceptualization, R.T., R.M. and M.C.; methodology, F.M.-S. and R.M.; validation, R.M., and M.C.; formal analysis, R.T.; investigation, R.T., R.M. and M.C.; resources, R.M.; data curation, R.T.; writing—original draft preparation, R.T.; writing—review and editing, R.T., F.M.-S., R.M. and M.C.; supervision, R.M. and M.C.; project administration, M.C.; funding acquisition, R.M. and M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Renan Tosin and Filipe Monteiro-Silva acknowledge Fundação para a Ciência e Tecnologia (FCT) PhD research grants Ref. SFRH/BD/145182/2019 and SFRD/BD/09136/2020. Rui Martins acknowledges the Fundação para a Ciência e Tecnologia (FCT) research contract grant (CEEIND/017801/2018).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rogiers, S.Y.; Greer, D.H.; Hatfield, J.M.; Orchard, B.A.; Keller, M. Mineral sinks within ripening grape berries (Vitis vinifera L.). Vitis-Geilweilerhof- 2006, 45, 115. [Google Scholar]
- Tewari, R.K.; Yadav, N.; Gupta, R.; Kumar, P. Oxidative Stress Under Macronutrient Deficiency in Plants. J. Soil Sci. Plant Nutr. 2021, 21, 832–859. [Google Scholar] [CrossRef]
- Bertoldi, D.; Larcher, R.; Bertamini, M.; Otto, S.; Concheri, G.; Nicolini, G. Accumulation and distribution pattern of macro- and microelements and trace elements in Vitis vinifera L. cv. Chardonnay berries. J. Agric. Food Chem. 2011, 59, 7224–7236. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K. Review: Application Of LIBS To Elemental Analysis And Mapping Of Plant Samples. At. Spectrosc. 2021, 42. [Google Scholar] [CrossRef]
- Arantes de Carvalho, G.G.; Bueno Guerra, M.B.; Adame, A.; Nomura, C.S.; Oliveira, P.V.; Pereira de Carvalho, H.W.; Santos, D.; Nunes, L.C.; Krug, F.J. Recent advances in LIBS and XRF for the analysis of plants. J. Anal. At. Spectrom. 2018, 33, 919–944. [Google Scholar] [CrossRef]
- Jull, H.; Künnemeyer, R.; Schaare, P. Nutrient quantification in fresh and dried mixtures of ryegrass and clover leaves using laser-induced breakdown spectroscopy. Precis. Agric. 2018, 19, 823–839. [Google Scholar] [CrossRef]
- He, Y.; Zhao, Y.; Zhang, C.; Li, Y.; Bao, Y.; Liu, F. Discrimination of Grape Seeds Using Laser-Induced Breakdown Spectroscopy in Combination with Region Selection and Supervised Classification Methods. Foods 2020, 9, 199. [Google Scholar] [CrossRef] [PubMed]
- Eilers, P.H.C. A Perfect Smoother. Anal. Chem. 2003, 75, 3631–3636. [Google Scholar] [CrossRef] [PubMed]
- Eilers, P.H.C. Parametric Time Warping. Anal. Chem. 2004, 76, 404–411. [Google Scholar] [CrossRef] [PubMed]
- OSCAR-Optical Science Center for Applied Research at Delaware State University LIBS database. Available online: https://oscar.desu.edu/libs/ (accessed on 20 May 2020).
- NIST Atomic Spectra Database Lines Form. Available online: https://www.physics.nist.gov/PhysRefData/ASD/lines_form.html (accessed on 20 May 2020).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).