1. Introduction
The humidity sensors are among the most used devices not only in the industry of heating, ventilation, and air conditioning (HVAC), but also in many different fields such as biomedicine, food processing, pharmaceuticals, meteorology, microelectronics, and agriculture. The humidity control allows operators to either preserve comfortable and healthy environments or to furnish the appropriate background for many industrial processes [
1]. For instance, the production, transportation, and storage of food and medicines requires controlled relative humidity values. Similarly, in the semiconductor and electronics industry, the performance of produced devices often depends on the humidity [
2,
3]. Examples of new application areas for humidity sensors are wearable devices, structural health monitoring, or robotic smart skins [
4,
5]. The required key feature for these emerging applications is flexibility, achieved through choosing suitable sensing layers and substrates.
In the present work, a flexible humidity sensor operating at room temperature was obtained by drop casting a graphene oxide (GO) water solution onto silver interdigitated electrodes, previously inkjet printed on sheets of bimatted polyester. The GO is a 2D material that is flexible, stretchable, thermally stable, mechanically strong, lightweight, and biocompatible. The thickness of a GO single atomic layer is approximately 1 nm, while the lateral length can reach micrometers. The potential of GO in designing efficient humidity sensors was first demonstrated by Yao et al. who developed a quartz crystal microbalance and a stress-based device [
6,
7]. An extremely sensitive GO-based capacitive sensor was fabricated by Bi et al., who demonstrated excellent performance of the sensor along with rapid response and recovery times and long-term stability [
8].
GO can be easily produced using the Hummers and Offeman method which achieves graphite exfoliation and oxidation using permanganate [
9]. However, the accurate and precise choice of experimental conditions is crucial because the sensing capability and electrical properties vary from case to case depending on the synthesis procedure, purity, presence of defects, or oxidation degree. We adopted a modified Hummers’ method, followed by an alkaline treatment with a water solution of KOH that is able to induce a partial deoxygenation. With respect to hydrazine and sodium borohydride, chemical reduction with KOH is less efficient but advantageous for sensor design allowing either the improvement of electrical conductivity or preservation of more oxygenated functional groups, which are the major adsorption sites for water molecules.
The proposed device showed high sensitivity, good stability, and a fast recovery time. For comparison, an analogous device was fabricated using a water solution of commercial GO. Due to the capability to produce repeatable responses at room temperature, the easy fabrication process, and the high elasticity and robustness, the described sensors could be easily integrated into wearable equipment.
2. Materials and Methods
2.1. GO Synthesis
All the reagents required to synthesize GO were purchased from Sigma-Aldrich (St. Louis, MO, USA) and used without further purification. A concentrated water solution of H2SO4 (23 mL at 96% by weight) was added to a flask containing graphite. The resulting mixture was cooled down using an ice bath and 3 g of KMnO4 was added in small aliquots under vigorous stirring. After removal of the ice bath, 140 mL of deionized water and 10 mL of H2O2 were slowly added. The GO brown-yellowish powder was separated by centrifugation (4000 rpm for 10 min) and was washed three times with a water solution of HCl (concentration of 10% by volume). Finally, an alkaline treatment was performed by washing the GO powder (100 mg) with a water solution of KOH (10 mL at 10% by weight).
The obtained dried powder were compressed into KBr pellets and FTIR spectra were acquired with a NicoletTM iS50 spectrometer (Thermo Fisher Scientific, Monza, Italy).
2.2. Chemiresistive Device Fabrication
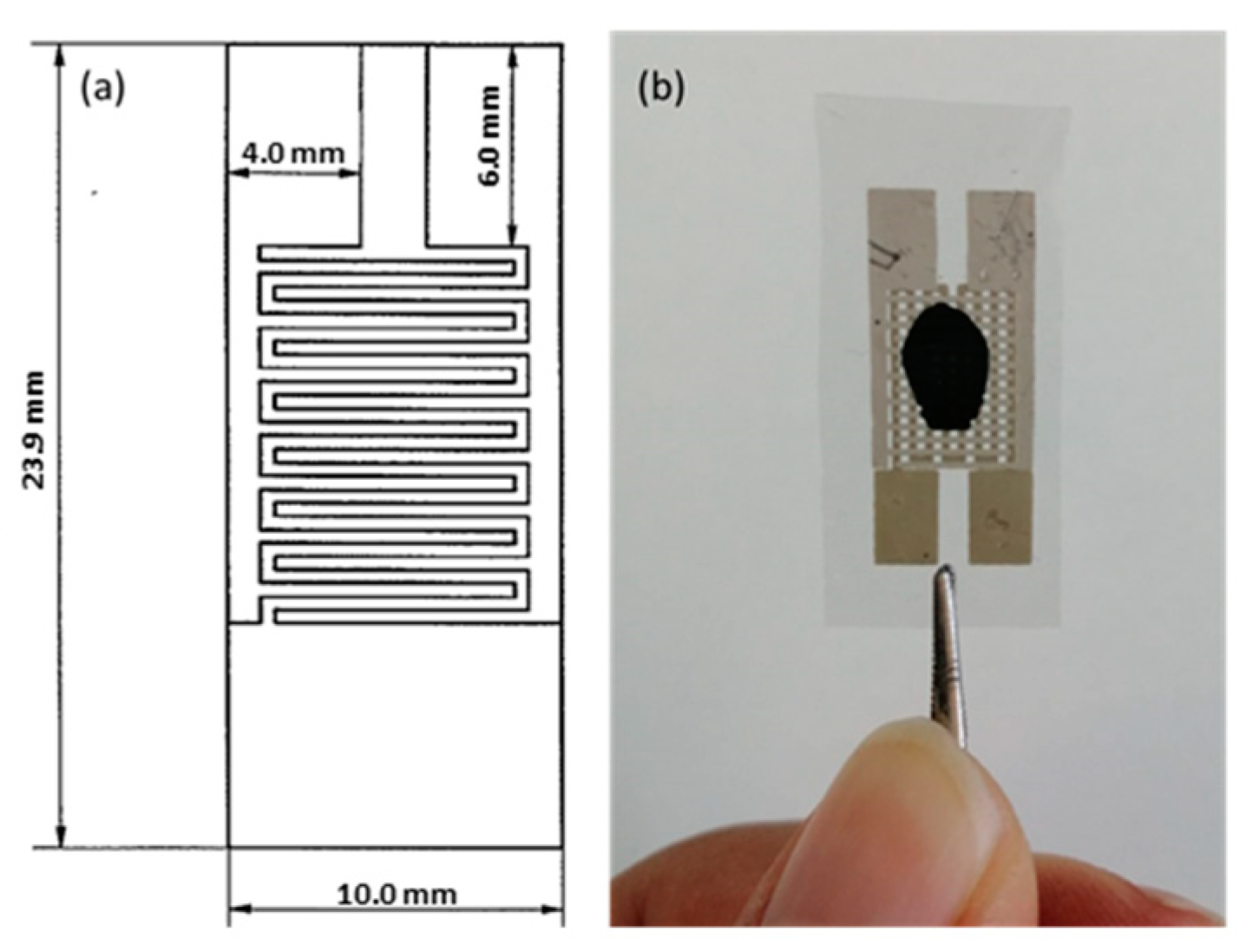
For device fabrication, sheets of bimatted polyester were aligned under a photomask, previously impressed by screen printing with a bromograph. A silver-based conductive ink was applied and cured a high temperature. Interdigitate electrodes with a line and span width of 0.4 mm were produced. Each electrode had 7 lines distributed on a total area of 11.2 × 10 mm.
Figure 1a shows a schematic diagram of the printed interdigitated electrodes. On the back of the polyester sheet, a heating circuit was inkjet printed using graphite-based ink. This circuit was used to stabilize the resistance values of the GO coatings immediately after deposition by heating to a temperature of 50 °C.
Two types of GO were tested: the first one was purchased from Sigma Aldrich and used without further purification (product number 777676), and the second one synthesized by the method described above. The dropped volume was 200 µL in both cases.
Figure 1b shows the GO-coated interdigitated electrodes.
2.3. Experimental Setup for Gas Sensing Measurements
The two devices were simultaneously located in a sealed stainless container with a volume of approximately 500 cc. Each vapor test was performed by passing a controlled flow of a carrier gas (dry air) in a glass cylinder containing water at room temperature. An additional dry air steam was used to tune the dilution of the saturated stream. Changes in the device resistance were measured at varying levels of relative humidity in the range from 15% to 70% by the volt-amperometric technique in the two-pole format using a multimeter (Agilent 34401A, Milano, Italy). The chemiresistor devices were automatically scanned by a switch system (Keithley 7001, Milano, Italy) equipped with a low-current scanner card (Keithley 7158, Milano, Italy) with a multiplex read-out. All data were acquired and stored in a PC-based workstation equipped with software compiled in Agilent-VEE using an USB-GPIB interface card (Agilent, 82357A, Milano, Italy). The relative humidity values were monitored using a Vaisala humidity and temperature probe. All the exposures to wet air were conducted at room temperature. The sensing cycle consisted of three steps: (i) a period of 60 min where the dry air flow reached a stable value of resistance; (ii) exposure to various relative humidity values for 10 min; and finally, (iii) a recovery time of 50 min under dry air flux to restore the starting resistance value.
3. Results and Discussion
The GO surface is characterized by the presence of several chemical groups such as carboxyl (−COOH), hydroxyl (−OH), epoxy (C−O−C), carbonyl (−C−OH), ketone (−C=O), and 5- and 6-membered ring lactols (O−C−O). These groups are the major sites of water interaction, mainly through hydrogen bond formation [
10].
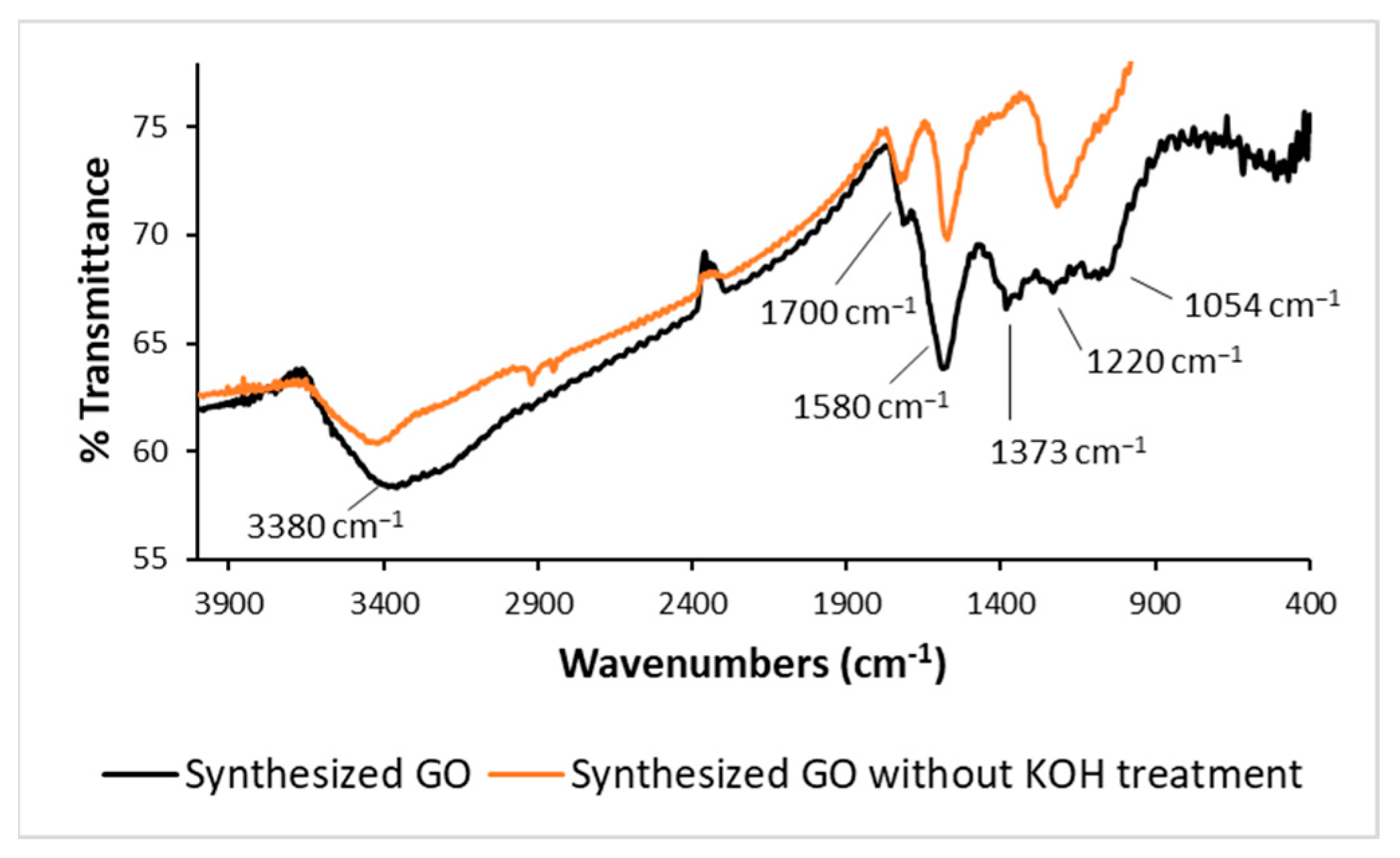
The presence of −OH groups was attested by the appearance of a characteristic broad absorption band at 3380 cm
−1 in the FTIR spectra shown in
Figure 2.
The effects of the basic treatment with a water solution of KOH clearly emerge in the FTIR spectra reported in
Figure 2 for synthesized GO after KOH treatment and for the same sample before basic cleavage. The peak at 1700 cm
−1, due to −C=O stretching vibration, reduced in intensity following basic treatment, while the peak at 1580 cm
−1, due to aromatic stretching vibration of C=C bonds, visibly increased. As expected for a reducing process, new sp
2 carbon atoms were introduced, extending the graphene moieties. The FTIR signals at 1373, 1220 and 1054 cm
−1 arise from −C−OH stretching, C−O−C stretching, and C−O stretching, respectively. According to the literature, the mild reduction process performed with a basic solution mainly induces the removal of carbonyl groups and the resulting GO preserves above all epoxide and hydroxyl groups [
11].
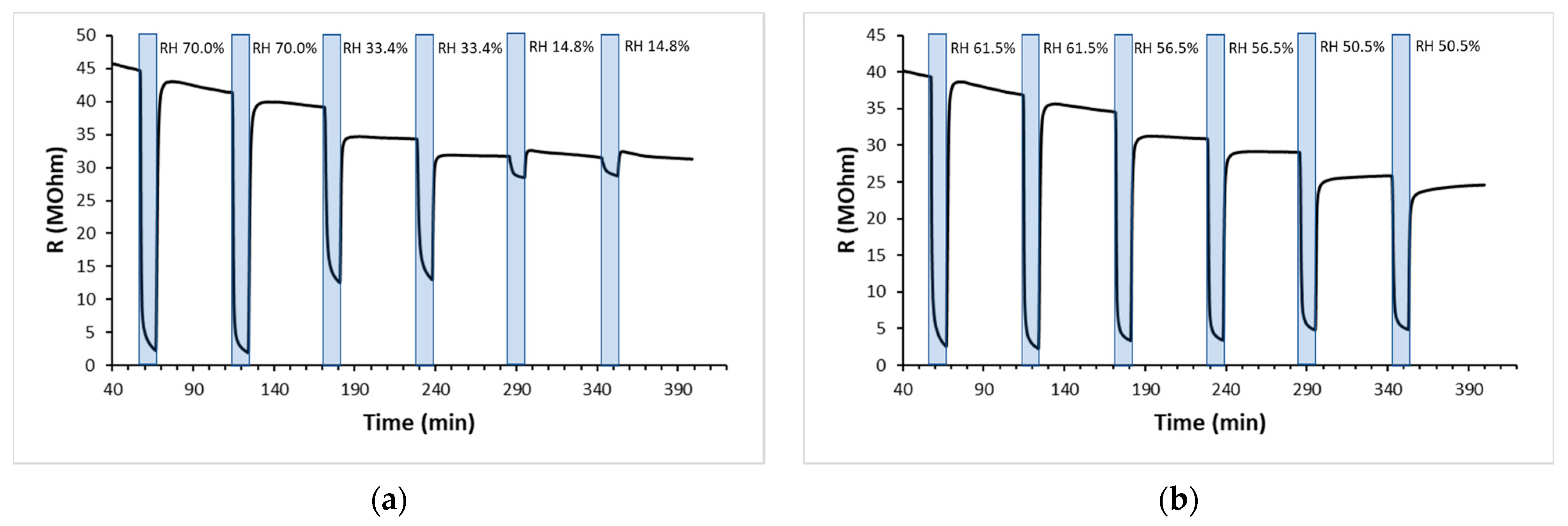
The synthesized partially reduced GO, deposited on the described flexible devices, was exposed to wet air in a relative humidity range of 15–70% at room temperature, and some typical responses are shown in
Figure 3. A drastic decrease in electrical resistance was observed with increasing relative humidity.
The responses were very reproducible and stable over time, but a partial recovery showed that the resistance values were lower than the starting resistance, indicating an incomplete removal of water molecules from the sensing layer.
The sensor responses were calculated using the following relationship:
where R
g and R
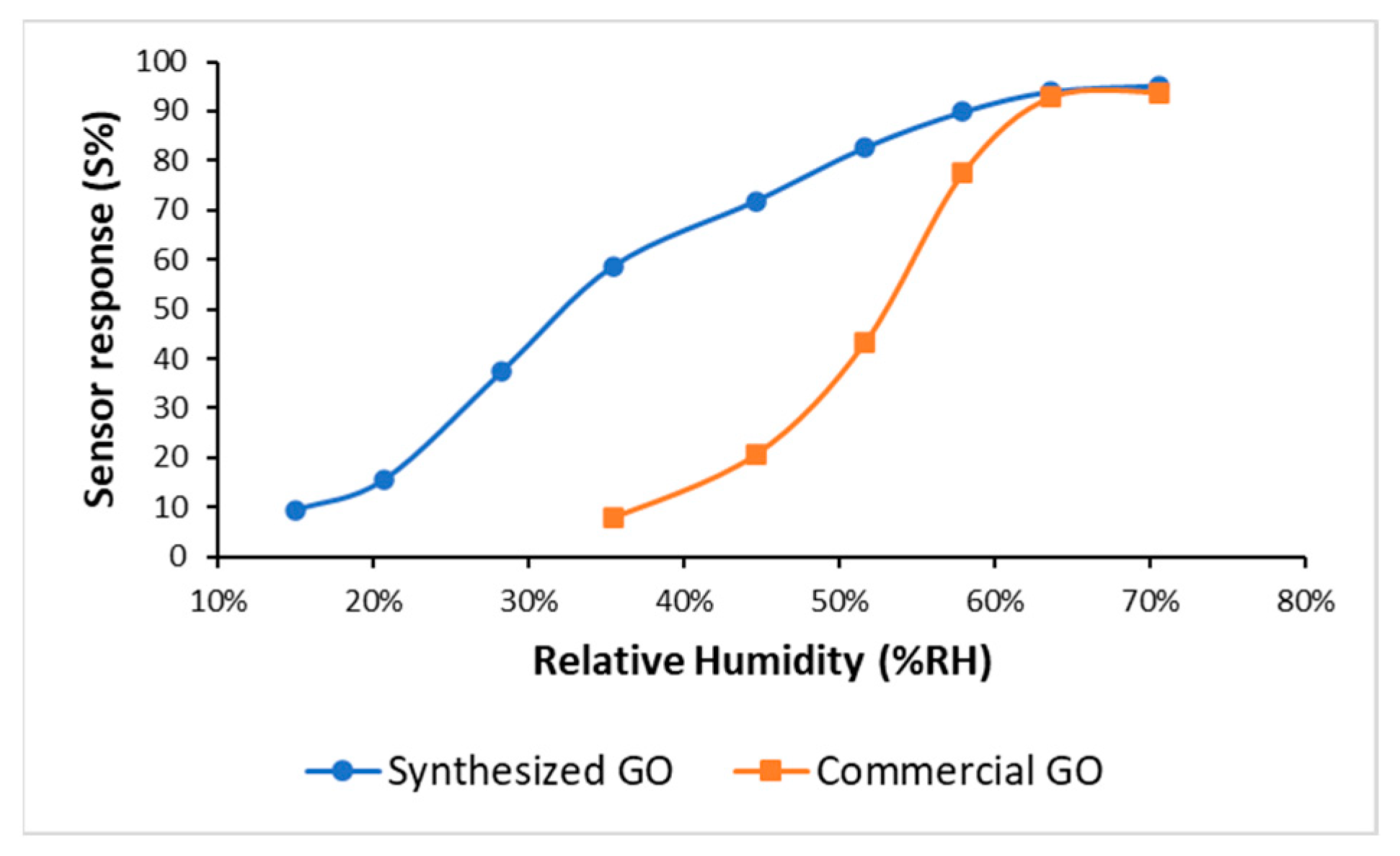
a are the electrical resistance measured upon exposure to wet air and dry air, respectively. In
Figure 4, the sensor responses of synthesized GO and commercial GO were compared. Higher sensitivities were measured with synthesized GO within the considered humidity range.
At low values of relative humidity, the increase in sensor responses with increasing relative humidity is mainly due to physiosorbed water molecules. In this regime, the amount of adsorbed water molecules is not enough to allow the hopping transfer of protons between adjacent hydroxyl groups [
7]. In addition, the formation of relatively strong hydrogen bonds with GO oxygenated groups restricts the mobility of adsorbed water molecules. Consequently, the measured GO resistances very high. At a higher relative humidity, the water molecules interact either with oxygenated groups on the GO surface or with other adsorbed water molecules forming a second adsorbed layer in which they preserve higher mobility. In this regime, the conductivity generated by the Grotthuss chain reaction (H
2O + H
3O
+ ⇆ H
3O
+ + H
2O) induces a drastic decrease in electrical resistance. At values of relative humidity higher than 65%, the sensitivity did not change significantly. In this regime, water molecules deeply penetrate between GO layers and the resulting increased interlayer distance negatively affects the resistance values [
12].
The recovery times, defined as the time required to return to 90% of the original resistance after the removal of wet air, were 40 and 70 s for the synthetized and commercial GO, respectively.
Although the responses of the sensors remained stable over time, a decrease in the GO baseline resistances was observed over six months, indicating structural and chemical changes in the active layer. In future, understanding the chemistry of GO exposed to external stimuli such as light, humidity, or temperature will be crucial in improving GO-based devices.
4. Conclusions
In summary, we have fabricated a chemiresistive flexible humidity sensor using a thin GO active layer. Being rich in oxygenated functional groups, such as hydroxyl and epoxy groups, the GO can easily capture water molecules from the external environment. When the water concentration is sufficiently high, the conductivity generated by the Grotthuss chain reaction induces a drastic resistance decrease in the GO thin film. Higher sensor performances in terms of sensitivity and recovery time were measured for the synthesized GO with respect to commercial GO.
The good sensitivity, the response repeatability at room temperature, the wide RH range detected, and the fast recovery time are the main advantages of the proposed humidity sensor. Furthermore, the flexibility, the easy fabrication process, the high elasticity, and robustness make the device easier to use in many emerging application areas, such as wearable devices, structural health monitoring, or robotic smart skins. The disadvantageous aspects are related to the deposition of the GO film, because dropping a water solution with a pipette does not allow a strict control of the thickness and roughness of the film. The conductivity of the resulting GO films significantly changed within different thicknesses. In addition, after the recovery run, the resistance values were lower than the starting resistance, indicating a partial removal of water molecules from the sensing layer. Furthermore, a decrease in the GO baseline resistances was observed over a period of six months, indicating structural and chemical changes in the active layer. Controlling the structure, chemical stability, and properties of GO by choosing suitable synthesis and purification procedures will be crucial in improving the performance of GO-based devices.