Evaluation of Bacterial Growth Ability and PHA Production Using Various Combinations of Fatty Acids †
Abstract
:1. Introduction
2. Methods
2.1. Evaluation of the Growth Ability of CYR1 Strain by a Combination of Various Fatty Acids
2.2. PHA Production by CYR1 Strain
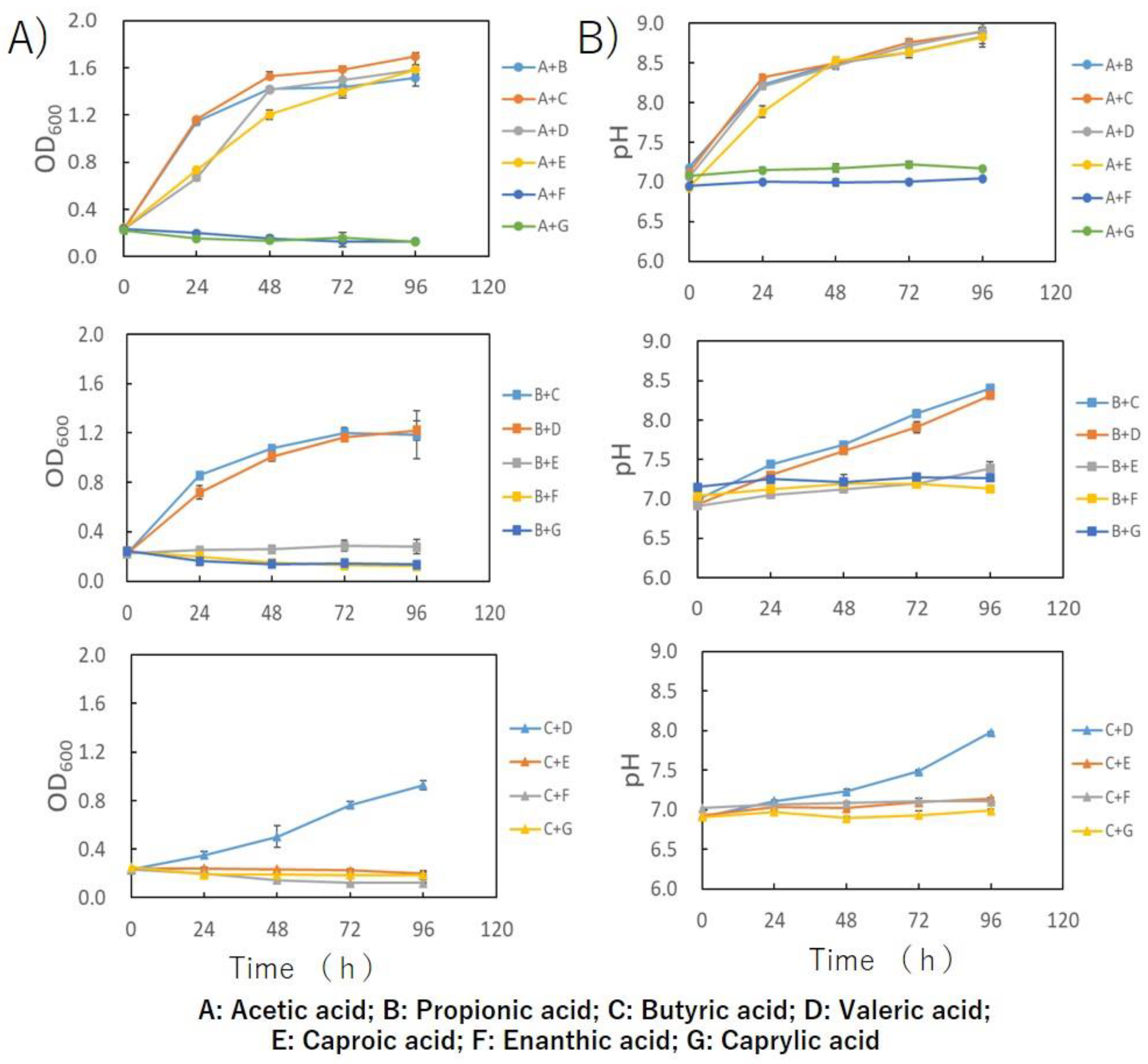
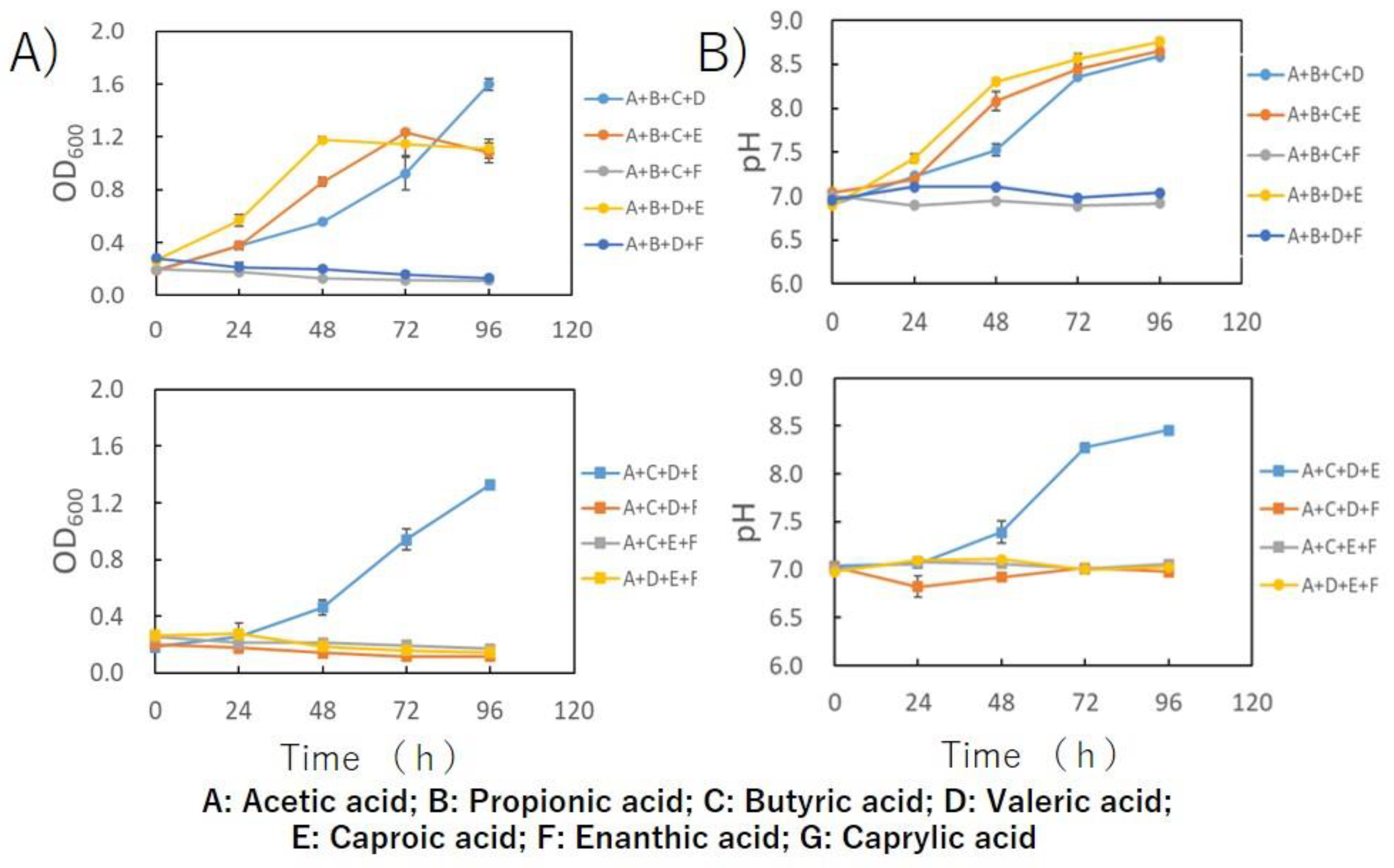
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Am. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, R.; Tanaka, A.; Takada, S. Marine Plastic Pollution: Dynamics of plastic debris in marine ecosystem and effect on marine organisms. Jpn. J. Ecol. 2016, 66, 51–68. [Google Scholar]
- Uchino, K.; Shiraki, M.; Saitou, M. Metabolism of poly-3-hydroxy butyric acid. Sci. J. Kanagawa Univ. 2009, 20, 283–286. (In Japanese) [Google Scholar]
- Reddy, M.V.; Yajima, Y.; Mawatari, Y.; Hoshino, T.; Chang, Y.C. Degradation and conversion of toxic compounds into useful bioplastics by Cupriavidus sp. CY-1: Relative expression of the PhaC gene under phenol and nitrogen stress. Green Chem. 2015, 17, 4560–4569. [Google Scholar] [CrossRef]
- Chang, Y.C.; Reddy, M.V.; Imura, K.; Onodera, R.; Kamada, N.; Sano, Y. Two-stage polyhydroxyalkanoates (PHA) production from cheese whey using Acetobacter pasteurianus C1 and Bacillus sp. CYR1. Bioengineering 2021, 8, 157. [Google Scholar] [CrossRef] [PubMed]
- Nath, A.; Dixit, M.; Bandiya, A.; Chavda, S.; Desai, A.J. Enhanced PHB production and scale up studies using cheese whey in fed batch culture of Methylobacterium sp. ZP24. Bioresour. Technol. 2008, 99, 5749–5755. [Google Scholar] [CrossRef] [PubMed]
- Yun, Z.; Yun, G.H.; Lee, H.S.; Yoo, T.U. The variation of volatile fatty acid compositions in sewer length, and its effect on the process design of biological nutrient removal. Wat. Sci. Technol. 2013, 67, 2753–2760. [Google Scholar] [CrossRef] [PubMed]
- Fernado, M.S.; Anton, K.; Peter, J.; Steven, P.; Nico, B.; Paul, L.; Alan, W. Production of polyhydoxyalkanoates in open, mixed cultures from a waste sludge stream containing high levels of soluble organics nitrogen and phosphorus. Water Res. 2010, 44, 5196–5211. [Google Scholar]
- Liliana, M.H.; Bronwyn, L.; Alan, W.; Steven, P. The evolution of polymer composition during PHA accumulation: The significance of reducing equivalents. Bioengineering 2017, 4, 20. [Google Scholar] [CrossRef]
- Reddy, M.V.; Mawatari, Y.; Yajima, Y.; Satoh, K.; Mohan, S.V.; Chang, Y.C. Production of poly-3-hydroxybutyrate(P3HB) and poly(3-hydroxbutyrate-co-3-hydroxyvalerate) P(3HB-co-3HV) from synthetic wastewater using Hydrogenophaga palleronii. Bioresour. Technol. 2016, 215, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.V.; Mawatari, Y.; Onodera, R.; Nakamura, Y.; Yajima, Y.; Chang, Y.C. Polyhydroxyalkanoates (PHA) production from synthetic waste using Pseudomonas pseudoflava: PHA synthase enzyme activity analysis from P. pseudoflava and P. palleronii. Bioresour. Technol. 2017, 234, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Jian, Y. Production of PHA from starchy wastewater via organic acid. J. Biotechnol. 2001, 86, 105–112. [Google Scholar]
- Munawar, K.; Munawar, M.; Simarani, K.; Mohamad, S.; Mohamad, A. Bioconversion of mixed free fatty acid to poly-3-hydroxyalkanoates by Pseudomonas putida BET001 and modeling of its fermentation in shake flasks. Electron. J. Biotechnol. 2016, 19, 50–55. [Google Scholar] [CrossRef]
- Reddy, M.V.; Mawatari, Y.; Yajima, Y.; Seki, C.; Hoshino, T.; Chang, Y.C. Poly-3-hydroxybutyrate (PHB) production from alkylphenols, mono and poly-aromatic hydrocarbons using Bacillus sp. CYR1: A new strategy for wealth from waste. Bioresour. Technol. 2015, 192, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.V.; Mawatari, Y.; Onodera, R.; Nakamura, Y.; Yajima, Y.; Chang, Y.C. Bacterial conversion of waste into polyhydroxybutyrate (PHB): A new approach of bio-circular economy for treating waste and energy generation. Bioresour. Technol. Rep. 2019, 7, 100246. [Google Scholar] [CrossRef]
- Watanabe, A. A Study on PHA Production Using Multiple Fatty Acids by Bacillus sp. CYR1 Strain. Bachelor’s Thesis, Muroran Institute of Technology, Hokkaido, Japan, 2020. (In Japanese). [Google Scholar]

| Substrates | CWM (g/L) | CDM (g/L) | PHA Production (g/L) | PHA Production (%CDM) |
|---|---|---|---|---|
| Acetic acid, Butyric acid | 2.07 | 0.38 | 0.158 | 41.80 |
| Acetic acid, Propionic acid, Caproic acid | 2.51 | 0.60 | 0.241 | 40.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.-C.; Reddy, M.V. Evaluation of Bacterial Growth Ability and PHA Production Using Various Combinations of Fatty Acids. Eng. Proc. 2023, 37, 9. https://doi.org/10.3390/ECP2023-14612
Chang Y-C, Reddy MV. Evaluation of Bacterial Growth Ability and PHA Production Using Various Combinations of Fatty Acids. Engineering Proceedings. 2023; 37(1):9. https://doi.org/10.3390/ECP2023-14612
Chicago/Turabian StyleChang, Young-Cheol, and M. Venkateswar Reddy. 2023. "Evaluation of Bacterial Growth Ability and PHA Production Using Various Combinations of Fatty Acids" Engineering Proceedings 37, no. 1: 9. https://doi.org/10.3390/ECP2023-14612
APA StyleChang, Y.-C., & Reddy, M. V. (2023). Evaluation of Bacterial Growth Ability and PHA Production Using Various Combinations of Fatty Acids. Engineering Proceedings, 37(1), 9. https://doi.org/10.3390/ECP2023-14612







