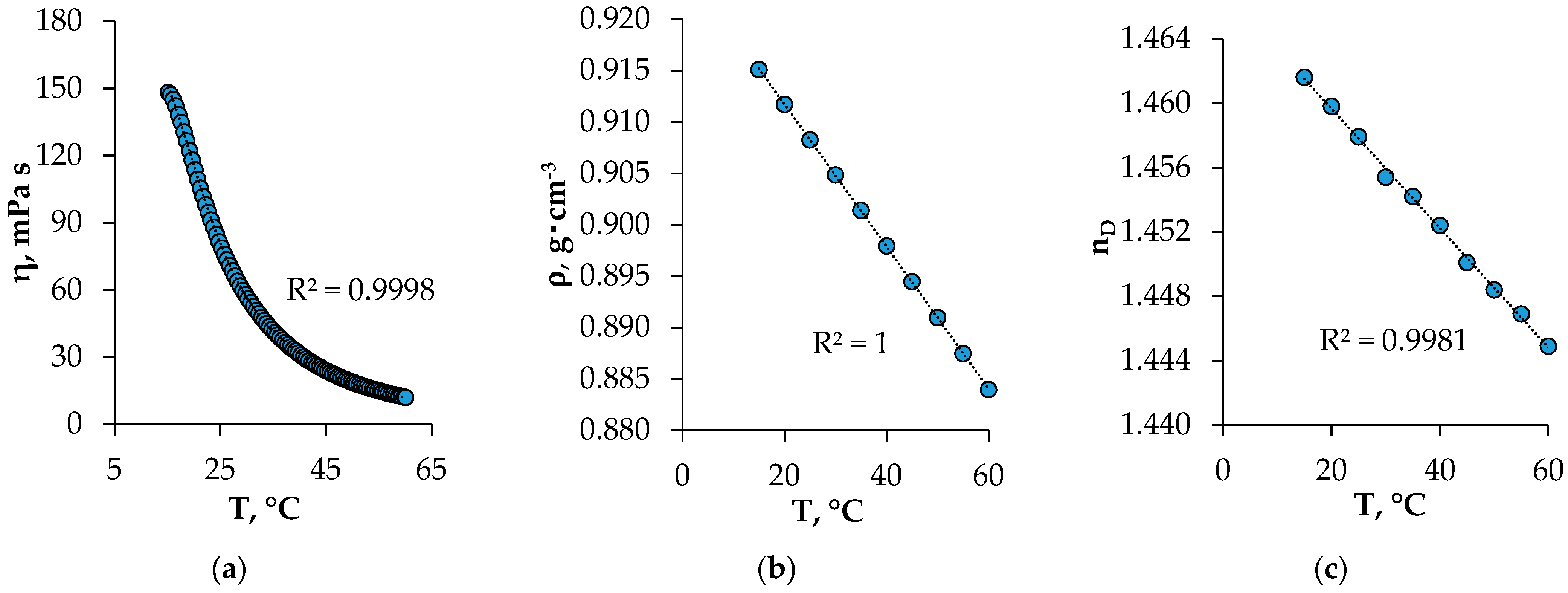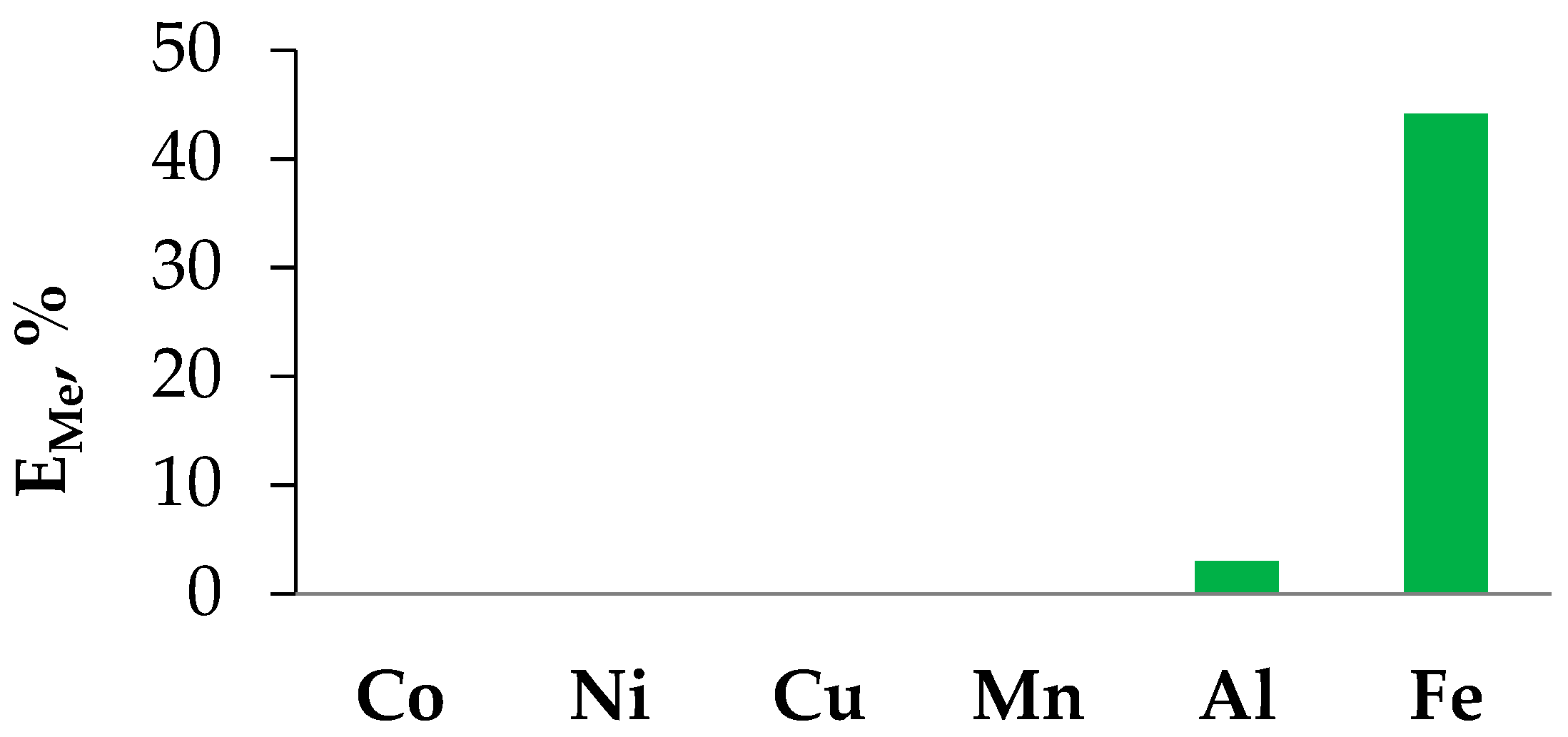Abstract
In this work, a new hydrophobic eutectic solvent (HES) based on bis(2,4,4-trimethylpentyl)phosphinic acid and menthol was synthesized and characterized for the first time. Important physical properties of the prepared HES, such as the density, viscosity, refractive index, as a function of temperature in the range 15–60 °C, were obtained. The new HES was applied for the extraction of Co, Ni, Cu, Mn, Al, and Fe from chloride solution. The extraction rate of Fe(III) reached 44%, while for other metals the extraction rate was not more than 3%. The potential possibility of the selective extraction of iron ions from an aqueous solution of a mixture of metals using the proposed HES was shown.
1. Introduction
In recent decades, there have been notable advances in the development of new extraction systems that eliminate the use of highly toxic organic substances (e.g., toluene), including systems with supercritical fluids [1], water-soluble polymers [2], ionic fluids [3,4], and deep eutectic solvents [5,6].
Much attention today is focused on a more detailed study of a so-called new class of solvents, deep eutectic solvents (DES), which were discovered in 2003 by Abbott et al. [7]. These mixtures melt at lower temperatures than the original components, allowing them to remain liquid at room temperature, even though the original compounds are solids. The formation of intermolecular interactions between DES components is one of the key factors in the formation of DES and a significant decrease in the melting temperature [6,8]. Controlling the strength of intermolecular interactions by selecting the components and their ratio in the mixture allows one to influence the physical and chemical properties of DES, which is important for their use in solving a specific problem.
Hydrophobic deep eutectic solvents have already proven to be effective extractants for the extraction and separation of organic and inorganic substances [9,10,11,12,13].
Note that in this paper, we use the term “hydrophobic eutectic solvent, HES” in order to have a wider choice of mixture compositions regardless of the lowest melting point. The aim of the present work is a detailed physical and chemical characterization of a hydrophobic eutectic solvent based on di(2,4,4-trimethylpentyl)phosphinic acid and menthol and evaluation of the possibility of its application as an extractant for the extraction of transition metal ions in the liquid–liquid system.
2. Materials and Methods
Bis(2,4,4-trimethylpentyl)phosphinic acid (BTMPPA, purity 85%, Cytec, Woodland Park, NJ, USA) and L-menthol (purity 99.5%, Chimmed, Moscow, Russia) of chemically pure grade were used without further purification.
2.1. Preparing of HES
The HES was prepared from a hydrogen bond acceptor (BTMPPA) and a donor (menthol) in a molar ratio of 1:1. The reagents weighed on an analytical balance (AND HR-100AZ, Tokyo, Japan) were placed into 50 mL plastic tubes. To form an HES, the test tubes were placed in a thermostated Enviro-Genie SI-1202 shaker (Scientific Industries, Inc., Bohemia, NY, USA, accuracy ± 0.2 °C) at a temperature of 70 °C and stirred at 35 rpm for 30 min until a liquid mixture was formed.
2.2. Characterization of HES
The phase diagram was determined by differential scanning calorimetry (DSC). DSC measurements were performed using a Mettler Toledo Instruments DSC 3 (Greifensee, Switzerland). Measurements were taken over three heating/cooling cycles across the temperature range from −90 to +50 °C, with a heating and cooling rate of 5 °C min−1.
The FTIR spectra in the range of 4000–600 cm–1 were recorded on a Shimadzu IRTracer-100 spectrometer (Kyoto, Japan). The 31P spectra were recorded in DMSOD6 on a Bruker Fourier 300 HD spectrometer (Billerica, MA, USA). The HES viscosity was measured on an Anton Paar Physica MCR301 rheometer (Graz, Austria) with a constant shear rate of 10 s−1. The HES density was determined on an Anton Paar DMA 1001 m (Graz, Austria) with a measurement accuracy of ±0.0001 g/cm3. The refractive index was determined on an Anton Paar Abbemat 3200 refractometer with a measurement accuracy of ±0.0001 (Graz, Austria).
2.3. Extraction of Metals with HES
All extraction experiments were carried out at a temperature of 25 °C and an atmospheric pressure of ~100 kPa in graduated centrifuge tubes with a thermostatically controlled shaker. The volume ratio of the aqueous phase to the HES phase was 2:1, respectively. The initial concentration of all the metal ions was 0.01 mol/L, and the initial pH value of the aqueous phase was 3.5. Graduated test tubes were placed in a shaker and stirred at a constant temperature during the entire process with a rotation speed of 35 rpm until thermodynamic equilibrium was established (15 min). After mixing, the samples were centrifuged at 2500 rpm for 5 min until complete phase separation in a SIA ELMI CM-6MT centrifuge (Riga, Latvia). The phases were then separated in separating funnels.
The concentration of metal ions in the initial solution and in the aqueous phase after extraction was determined using a spectrophotometric method based on obtaining complexes of metal ions with 4-(2-pyridylazo)resorcinol absorption in the visible spectrum with the following wavelengths (nm): Cu (508), Ni (495), Mn (502), and Co (510) in the Cary-60 spectrophotometer (wavelength accuracy ± 0.06 nm). The concentration of Fe(III) ions after extraction was determined by spectrophotometry in the visible region at a wavelength of 420 nm using sulfosalicylic acid as an indicator relative to the blank solutions. The content of Al(III) in the aqueous phase after extraction was determined spectrophotometrically in the visible region (λ = 553 nm) with xylenol orange relative to water as an indicator.
The presented experimental data are the result of a series of experiments; they were processed with methods of mathematical statistics. The error in determining the concentrations was less than 5%.
3. Results and Discussion
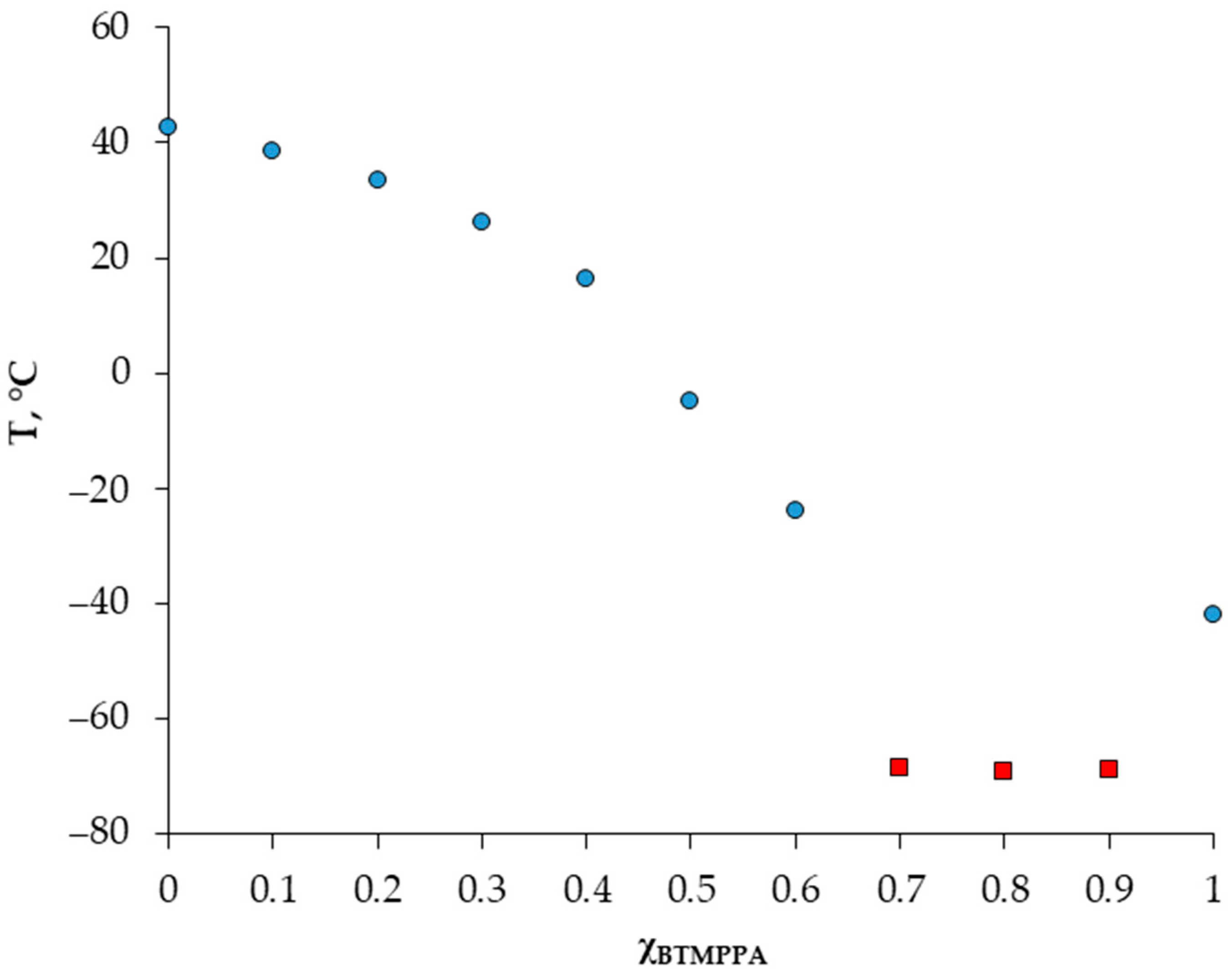
A detailed investigation of the solid–liquid phase behavior of the Cyanex 272/menthol system was made using DSC. The presence of a eutectic point on the solid–liquid diagram is a defining characteristic of a deep eutectic solvent. Figure 1 shows the dependence of the melting temperature of the BTMPPA/menthol mixture on its composition. In the range of compositions (ꭓBTMPPA = 0.7–0.9), we obtained points corresponding to the glass transition temperatures of the mixtures, and the DSC curves of these compositions upon heating were almost featureless. A similar effect was observed in a number of works [14,15]. For the extraction experiment, compositions where ꭓBTMPPA ≥ 0.5 were selected.
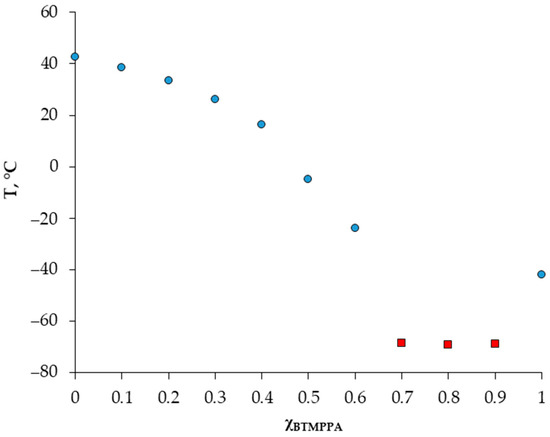
Figure 1.
Solid–liquid phase diagrams of the BTMPPA/menthol mixture. Blue dots—melting temperature; red squares—glass transition temperature.
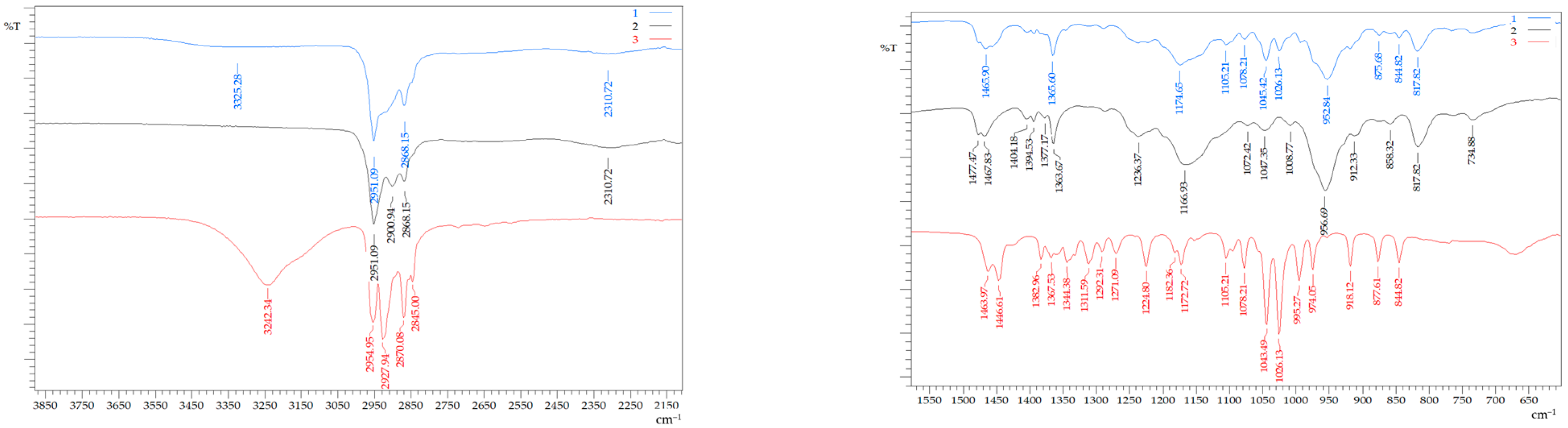
FTIR spectroscopy was used to confirm the formation of a hydrophobic eutectic solvent with the ATR technique. The FTIR spectra of individual substances and HES were obtained, in which there were absorption bands shifted relative to the bands of the initial components (Figure 2). A characteristic shift was observed in the area of the absorption band related to the stretching vibrations of the –O–H group of menthol from 3242.34 to 3325.28 cm−1. During the formation of the HES, the absorption band of the stretching vibrations of the P=O group of BTMPPA shifted from 1166.93 to 1174.65 cm−1. Thus, these features of the HES spectrum indicated the formation of a hydrogen bond between the menthol hydroxyl group and the oxygen atom of the P=O group. It should be noted that a slight shift of the characteristic absorption band of P–O–C from 956.69 to 952.84 cm−1 is most likely due to the absence of the interaction of hydrogen bonds between the organophosphoric acid molecules during the formation of dimers [16].
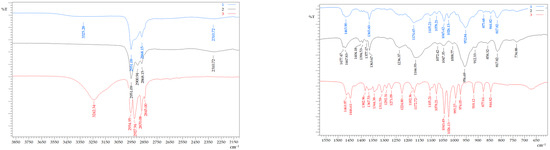
Figure 2.
FTIR spectrum of HES—1, BTMPPA—2, and menthol—3.
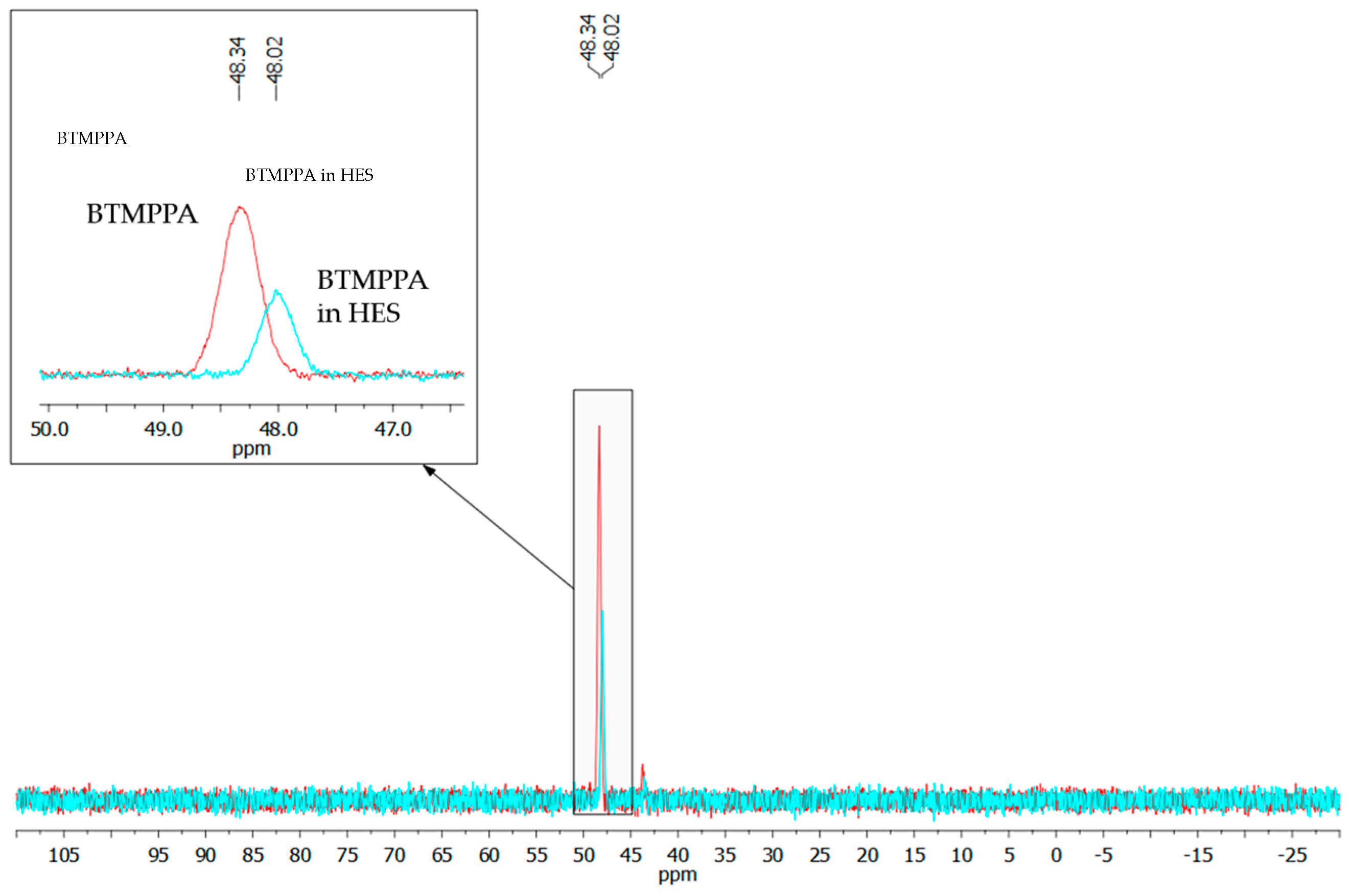
The assumed mechanism of HES formation was also confirmed by the characteristic shift of the phosphorus atom signal at the P=O double bond of BTMPPA from 48.34 to 48.02 ppm (Figure 3). The shift of this signal in the strong field during HES formation confirmed the shift in the electron density toward the P=O double bond due to the fact that it acts as a hydrogen bond acceptor. Probably, such a shift of the signal is also associated with the destruction of dialkylphosphinic acid dimers.
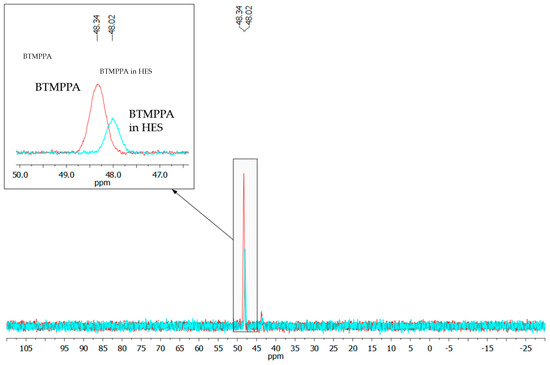
Figure 3.
31P NMR spectra of BTMPPA and BTMPPA/menthol HES.
The viscosity, density, and refractive index values of the HES ranged from 15 to 60 °C (Figure 4). These properties play an important role in mass transfer processes, affecting the emulsification and the ease of phase separation in the extraction process. The viscosity of the obtained HES, as expected, decreased with the increasing temperature. Under the conditions of the extraction experiment, the viscosity of the HES was 78.68 mPa s, which was less than the viscosity of pure BTMPPA (142 mPa s) [17]. The use of menthol as a component of HES has a favorable effect on the physical properties of HES, which makes it possible to use it as an extractant. The density and refractive index of the studied HES decreased with the increasing temperature, which correlated with the literature data [18,19].
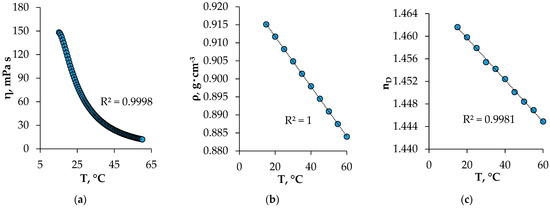
Figure 4.
Viscosity—(a), density—(b), and refractive index—(c) of the synthesized HES at different temperatures.
Based on the experimentally obtained data on the physical properties of the hydrophobic eutectic solvent based on BTMPPA and menthol for experimental studies of the extraction of transition metal ions in the liquid–liquid system, we chose a molar ratio of the acceptor and hydrogen bonding donor equal to 1:1. Several metals (Co, Ni, Cu, Mn, Al, and Fe) were chosen as model objects, which are most often included in the components of chemical current sources actively used today [20]. Based on the data presented in Figure 5, it can be concluded that the proposed HES can be used for the selective extraction of iron(III) from chloride-containing dilute solutions. A detailed study of the interphase distribution of transition metal ions in the extraction system with HES BTMPPA/menthol will be carried out in a separate study.
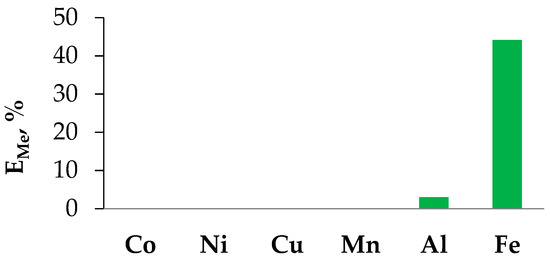
Figure 5.
Extraction rate of various metal chloride salts (mono-elemental solutions, [Me] = 0.01 M, pH = 1.5) in the extraction system with the HES.
Author Contributions
Conceptualization, I.V.Z. and A.V.K.; methodology, I.V.Z.; software, N.A.M.; validation, Y.A.Z. and A.A.V.; formal analysis, Y.A.Z.; investigation, I.V.Z.; resources, A.A.V.; data curation, Y.A.Z.; writing—original draft preparation, I.V.Z. and N.A.M.; writing—review and editing, Y.A.Z.; visualization, A.A.V.; supervision, I.V.Z. and Y.A.Z.; project administration, A.A.V.; funding acquisition, A.A.V. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by grant No. 20-13-00387 from Russian Science Foundation, https://rscf.ru/en/project/20-13-00387; (accessed on 15 May 2023).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Eckert, C.A.; Knutson, B.L.; Debenedetti, P.G. Supercritical Fluids as Solvents for Chemical and Materials Processing. Nature 1996, 383, 313–318. [Google Scholar] [CrossRef]
- Zinov’eva, I.V.; Zakhodyaeva, Y.A.; Voshkin, A.A. Extraction of Monocarboxylic Acids from Diluted Solutions with Polyethylene Glycol. Theor. Found. Chem. Eng. 2019, 53, 871–874. [Google Scholar] [CrossRef]
- Freire, M.G.; Cláudio, A.F.M.; Araújo, J.M.M.; Coutinho, J.A.P.; Marrucho, I.M.; Lopes, J.N.C.; Rebelo, L.P.N. Aqueous Biphasic Systems: A Boost Brought about by Using Ionic Liquids. Chem. Soc. Rev. 2012, 41, 4966. [Google Scholar] [CrossRef] [PubMed]
- Asrami, M.R.; Tran, N.N.; Nigam, K.D.P.; Hessel, V. Solvent Extraction of Metals: Role of Ionic Liquids and Microfluidics. Sep. Purif. Technol. 2021, 262, 118289. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Xue, Z.; Mu, T. Eutectics: Formation, Properties, and Applications. Chem. Soc. Rev. 2021, 50, 8596–8638. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep Eutectic Solvents Formed between Choline Chloride and Carboxylic Acids: Versatile Alternatives to Ionic Liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Fang, C.; Yang, L.; Li, K.; Zhu, K.; Liu, G.; Chen, J. The Novel Ionic Liquid and Its Related Self-Assembly in the Areas of Energy Storage and Conversion. Small Sci. 2022, 2, 2200048. [Google Scholar] [CrossRef]
- Kozhevnikova, A.V.; Zinov’eva, I.V.; Zakhodyaeva, Y.A.; Baranovskaya, V.B.; Voshkin, A.A. Application of Hydrophobic Deep Eutectic Solvents in Extraction of Metals from Real Solutions Obtained by Leaching Cathodes from End-of-Life Li-Ion Batteries. Processes 2022, 10, 2671. [Google Scholar] [CrossRef]
- Dwamena, A. Recent Advances in Hydrophobic Deep Eutectic Solvents for Extraction. Separations 2019, 6, 9. [Google Scholar] [CrossRef]
- Aşçı, Y.S.; Lalikoglu, M. Development of New Hydrophobic Deep Eutectic Solvents Based on Trioctylphosphine Oxide for Reactive Extraction of Carboxylic Acids. Ind. Eng. Chem. Res. 2021, 60, 1356–1365. [Google Scholar] [CrossRef]
- Mai, Y.; Xian, X.; Hu, L.; Zhang, X.; Zheng, X.; Tao, S.; Lin, X. Liquid–Liquid Extraction of Levulinic Acid from Aqueous Solutions Using Hydrophobic Tri-n-Octylamine/Alcohol-Based Deep Eutectic Solvent. Chin. J. Chem. Eng. 2023, 54, 248–256. [Google Scholar] [CrossRef]
- Wazeer, I.; Hizaddin, H.F.; Hashim, M.A.; Hadj-Kali, M.K. An Overview about the Extraction of Heavy Metals and Other Critical Pollutants from Contaminated Water via Hydrophobic Deep Eutectic Solvents. J. Environ. Chem. Eng. 2022, 10, 108574. [Google Scholar] [CrossRef]
- Gilmore, M.; McCourt, É.N.; Connolly, F.; Nockemann, P.; Swadźba-Kwaśny, M.; Holbrey, J.D. Hydrophobic Deep Eutectic Solvents Incorporating Trioctylphosphine Oxide: Advanced Liquid Extractants. ACS Sustain. Chem. Eng. 2018, 6, 17323–17332. [Google Scholar] [CrossRef]
- Alhadid, A.; Mokrushina, L.; Minceva, M. Formation of Glassy Phases and Polymorphism in Deep Eutectic Solvents. J. Mol. Liq. 2020, 314, 113667. [Google Scholar] [CrossRef]
- Regel-Rosocka, M.; Staszak, K.; Wieszczycka, K.; Masalska, A. Removal of Cobalt(II) and Zinc(II) from Sulphate Solutions by Means of Extraction with Sodium Bis(2,4,4-Trimethylpentyl)Phosphinate (Na-Cyanex 272). Clean Technol. Environ. Policy 2016, 18, 1961–1970. [Google Scholar] [CrossRef]
- Biswas, R.K.; Singha, H.P. Densities, Viscosities and Excess Properties of Bis-2,4,4-Trimethylpentylphosphinic Acid (Cyanex 272)+diluent Binary Mixtures at 298.15 K and Atmospheric Pressure. J. Mol. Liq. 2007, 135, 179–187. [Google Scholar] [CrossRef]
- Nowosielski, B.; Jamrógiewicz, M.; Łuczak, J.; Tercjak, A.; Warmińska, D. Effect of Temperature and Composition on Physical Properties of Deep Eutectic Solvents Based on 2-(Methylamino)Ethanol—Measurement and Prediction. J. Mol. Liq. 2023, 371, 121069. [Google Scholar] [CrossRef]
- Milevskii, N.A.; Zinov’eva, I.V.; Zakhodyaeva, Y.A.; Voshkin, A.A. Separation of Li(I), Co(II), Ni(II), Mn(II), and Fe(III) from Hydrochloric Acid Solution Using a Menthol-Based Hydrophobic Deep Eutectic Solvent. Hydrometallurgy 2022, 207, 105777. [Google Scholar] [CrossRef]
- Miao, Y.; Liu, L.; Zhang, Y.; Tan, Q.; Li, J. An Overview of Global Power Lithium-Ion Batteries and Associated Critical Metal Recycling. J. Hazard. Mater. 2022, 425, 127900. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).