Optimization Study for Desorption of Arsenic and Regeneration Performance on Magnetic Carbon Xerogels for Environmental Sustainability †
Abstract
:1. Introduction
2. Methods
2.1. Synthesis of Magnetic Carbon Xerogels
2.2. The Effect of Carbonization Temperatures to Obtain the Optimum Conditions
2.3. Characterization of Materials
2.4. The Kinetics of Arsenic Adsorption
2.5. The Optimal Condition for the Arsenate Desorption Process Using an RSM
2.6. Arsenic Adsorbent Regeneration
3. Results and Discussion
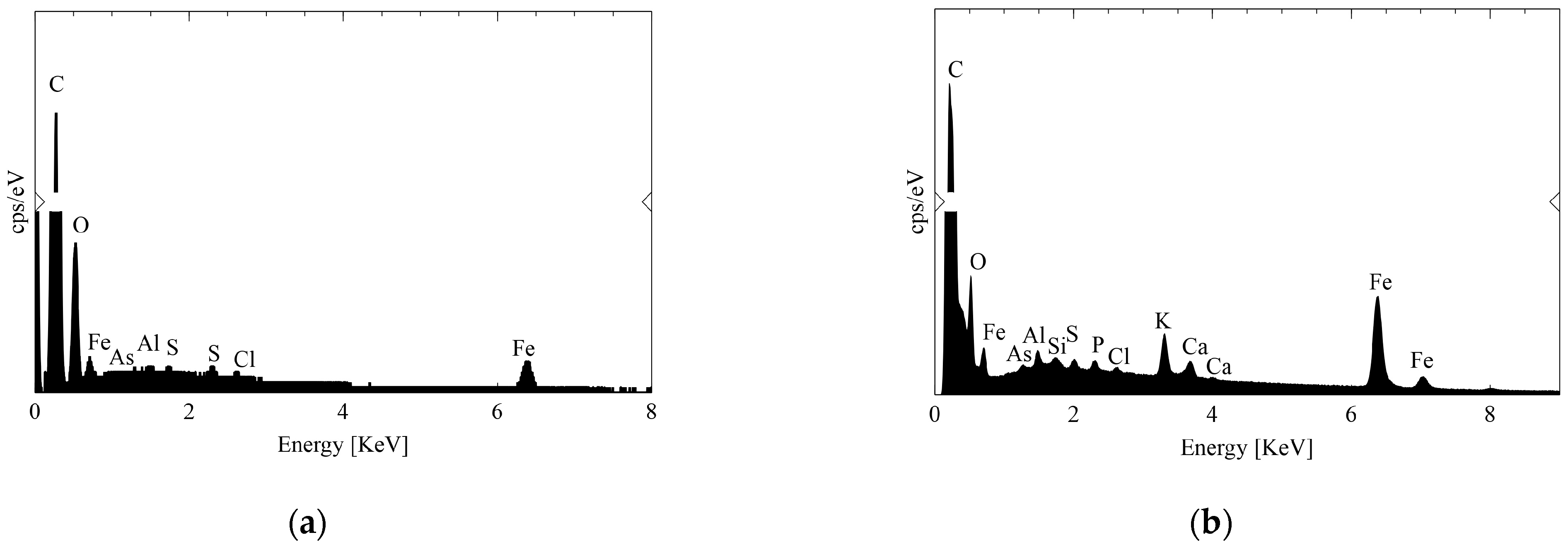
3.1. Characterization of Magnetic Carbon Xerogels
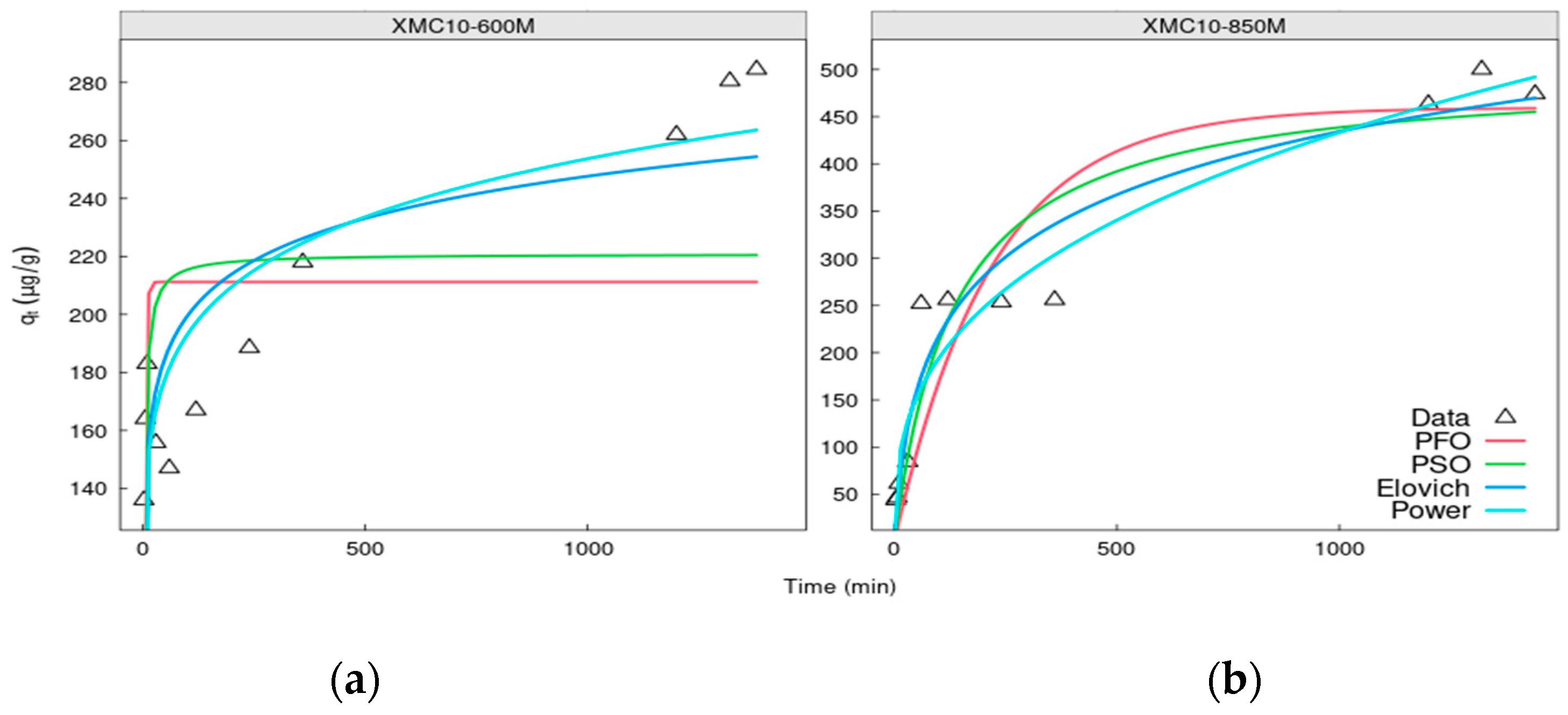
3.2. The Kinetics of Arsenic Adsorption
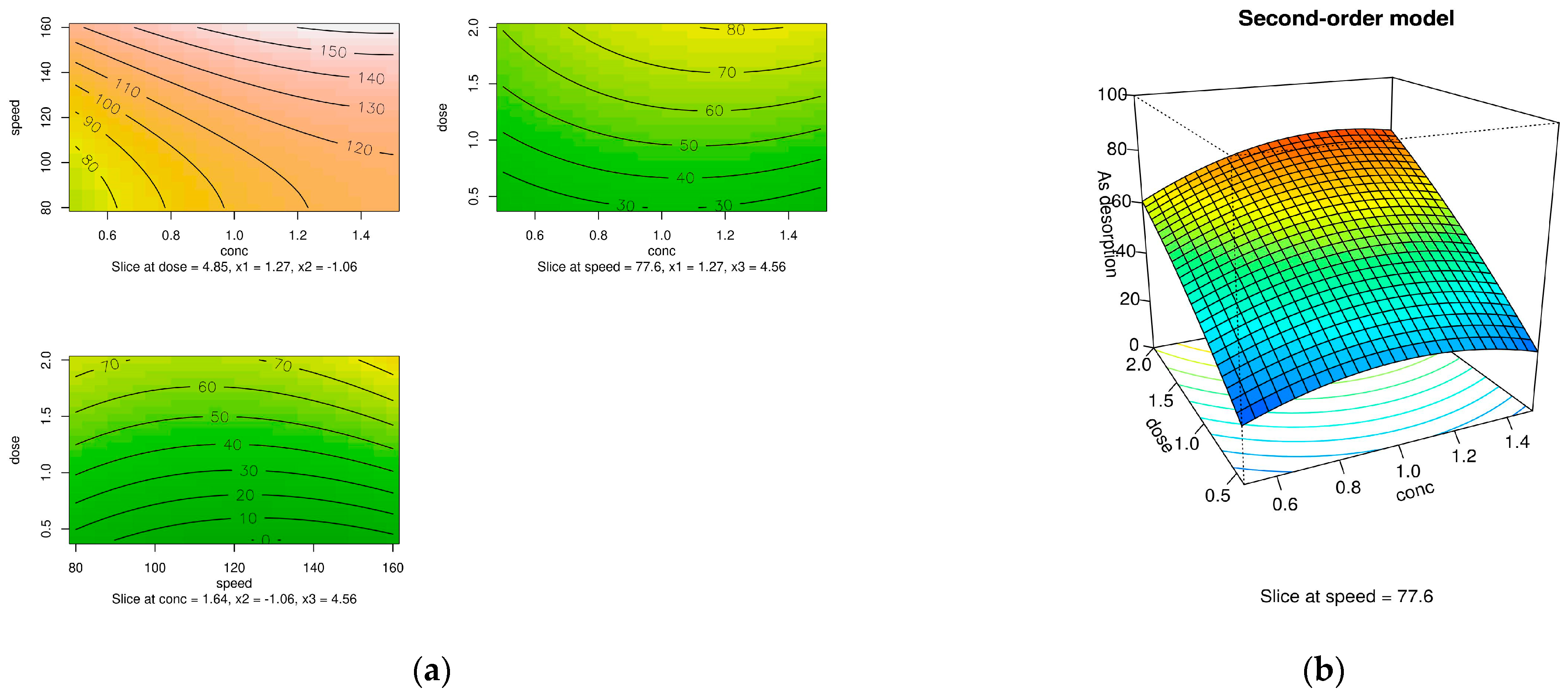
3.3. The Optimal Condition for the Arsenate Desorption Process Using an RSM
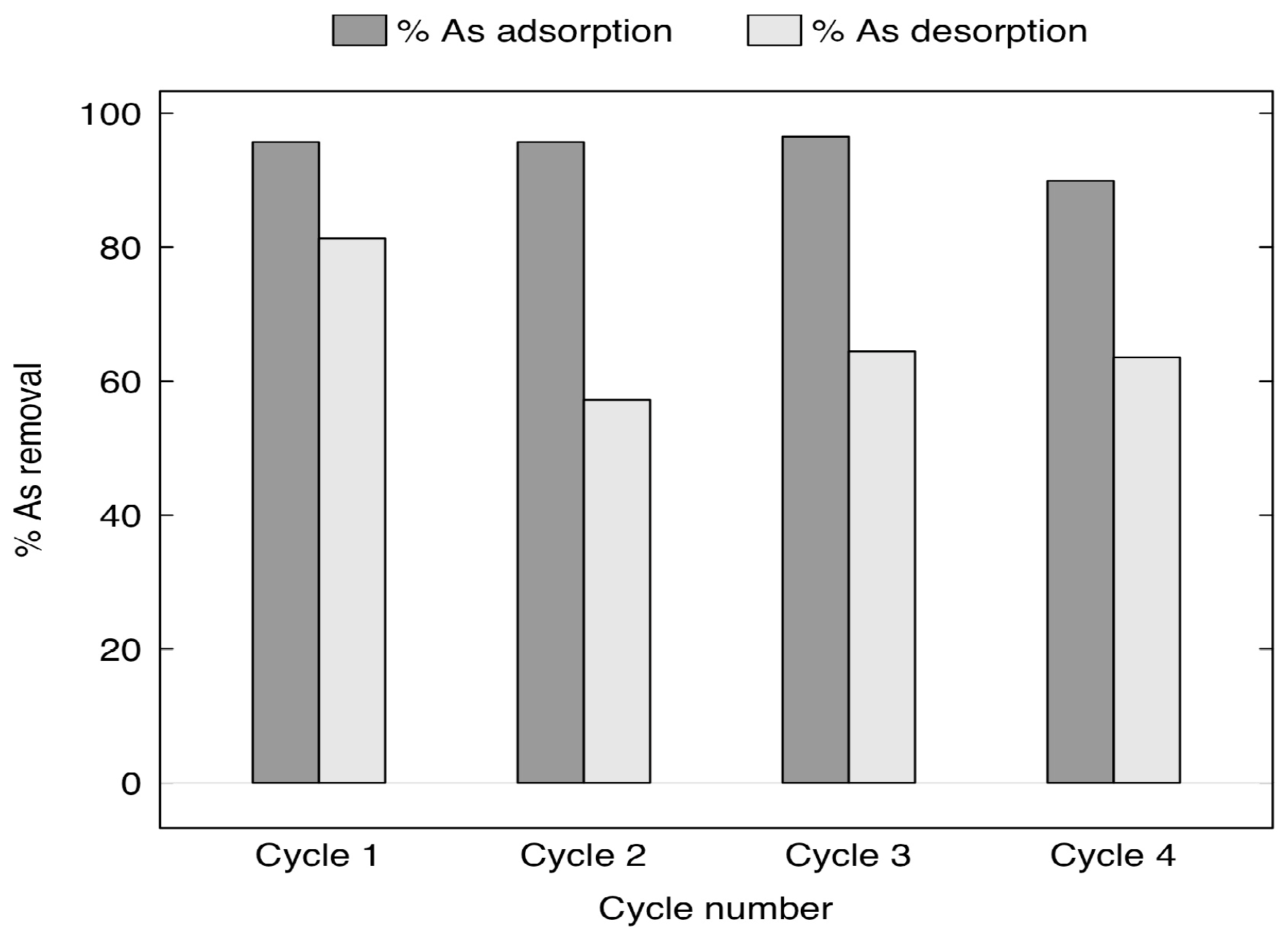
3.4. Arsenic Adsorbent Regeneration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, R.; Singh, S.; Parihar, P.; Singh, V.P.; Prasad, S.M. Arsenic contamination, consequences and remediation techniques: A review. Ecotoxicol. Environ. Saf. 2015, 112, 247–270. [Google Scholar] [CrossRef] [PubMed]
- Valskys, V.; Hassan, H.R.; Wołkowicz, S.; Satkūnas, J.; Kibirkštis, G.; Ignatavičius, G. A review on detection techniques, health hazards and human health risk assessment of arsenic pollution in soil and groundwater. Minerals 2022, 12, 1326. [Google Scholar] [CrossRef]
- Bibi, S.; Kamran, M.A.; Sultana, J.; Farooqi, A. Occurrence and methods to remove arsenic and fluoride contamination in water. Environ. Chem. Lett. 2017, 15, 125–149. [Google Scholar] [CrossRef]
- Nisticò, R. A synthetic guide toward the tailored production of magnetic iron oxide nanoparticles. Bol. la Soc. Esp. Ceram. y Vidr. 2020, 60, 29–40. [Google Scholar] [CrossRef]
- Perdigoto, M.; Martins, R.; Rocha, N.; Quina, M.; Gando-Ferreira, L.; Patrício, R.; Durães, L. Application of hydrophobic silica based aerogels and xerogels for removal of toxic organic compounds from aqueous solutions. J. Colloid Interface Sci. 2012, 380, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Li, W.; Song, Y. Preparation of SiO2–ZrO2 xerogel and its application for the removal of organic dye. J. Sol-Gel Sci. Technol. 2018, 86, 175–186. [Google Scholar] [CrossRef]
- Strachowski, P.; Fronczak, M.; Olechno, E.; Kowalik, M.; Kiciński, W.; Kaszuwara, W.; Bystrzejewski, M. Magnetic organic xerogels: Efficient adsorbents for the removal of heavy metal ions from aqueous solutions. New J. Chem. 2018, 42, 7073–7082. [Google Scholar] [CrossRef]
- Luzny, R.; Ignasiak, M.; Walendziewski, J.; Stolarski, M. Heavy metal ions removal from aqueous solutions using carbon aerogels and xerogels. Chemik 2014, 68, 544–553. [Google Scholar]
- Gore, P.; Khraisheh, M.; Kandasubramanian, B. Nanofibers of resorcinol–formaldehyde for effective adsorption of As (III) ions from mimicked effluents. Environ. Sci. Pollut. Res. 2018, 25, 11729–11745. [Google Scholar] [CrossRef]
- Verma, N.K.; Khare, P.; Verma, N. Synthesis of iron-doped resorcinol formaldehyde-based aerogels for the removal of Cr(VI) from water. Green Process. Synth. 2015, 4, 37–46. [Google Scholar] [CrossRef]
- Khamkure, S.; Bustos-Terrones, V.; Benitez-Avila, N.J.; Cabello-Lugo, M.F.; Gamero-Melo, P.; Garrido-Hoyos, S.E.; Esparza-Schulz, J.M. Effect of Fe3O4 nanoparticles on magnetic xerogel composites for enhanced removal of fluoride and arsenic from aqueous solution. Water Sci. Eng. 2022, 15, 305–317. [Google Scholar] [CrossRef]
- Embaby, M.A.; Moniem, S.M.A.; Fathy, N.A.; El-Kady, A.A. Nanocarbon hybrid for simultaneous removal of arsenic, iron and manganese ions from aqueous solutions. Heliyon 2021, 7, e08218. [Google Scholar] [CrossRef] [PubMed]
- Sikora, P.; Horszczaruk, E.; Cendrowski, K.; Mijowska, E. The influence of nano-Fe3O4 on the microstructure and mechanical properties of cementitious composites. Nanoscale Res. Lett. 2016, 11, 182. [Google Scholar] [CrossRef] [PubMed]

| Factors | Coding Factors | Low (−1) | Center (0) | High (1) |
|---|---|---|---|---|
| Concentration of KOH solution (M) | 0.5 | 1 | 1.5 | |
| Orbital shaker speed (rpm) | 80 | 120 | 160 | |
| Spent adsorbent dose (g/L) | 0.4 | 1.2 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khamkure, S.; Bustos-Terrones, V.; Torrecilla-Valle, A.; Gamero-Melo, P.; Reyes-Rosas, A.; Vargas-Gutiérrez, G.; Garrido-Hoyos, S.-E. Optimization Study for Desorption of Arsenic and Regeneration Performance on Magnetic Carbon Xerogels for Environmental Sustainability. Eng. Proc. 2023, 37, 34. https://doi.org/10.3390/ECP2023-14653
Khamkure S, Bustos-Terrones V, Torrecilla-Valle A, Gamero-Melo P, Reyes-Rosas A, Vargas-Gutiérrez G, Garrido-Hoyos S-E. Optimization Study for Desorption of Arsenic and Regeneration Performance on Magnetic Carbon Xerogels for Environmental Sustainability. Engineering Proceedings. 2023; 37(1):34. https://doi.org/10.3390/ECP2023-14653
Chicago/Turabian StyleKhamkure, Sasirot, Victoria Bustos-Terrones, Arael Torrecilla-Valle, Prócoro Gamero-Melo, Audberto Reyes-Rosas, Gregorio Vargas-Gutiérrez, and Sofía-Esperanza Garrido-Hoyos. 2023. "Optimization Study for Desorption of Arsenic and Regeneration Performance on Magnetic Carbon Xerogels for Environmental Sustainability" Engineering Proceedings 37, no. 1: 34. https://doi.org/10.3390/ECP2023-14653
APA StyleKhamkure, S., Bustos-Terrones, V., Torrecilla-Valle, A., Gamero-Melo, P., Reyes-Rosas, A., Vargas-Gutiérrez, G., & Garrido-Hoyos, S.-E. (2023). Optimization Study for Desorption of Arsenic and Regeneration Performance on Magnetic Carbon Xerogels for Environmental Sustainability. Engineering Proceedings, 37(1), 34. https://doi.org/10.3390/ECP2023-14653








