Supercritical Fluid Extraction as a Potential Extraction Technique for the Food Industry †
Abstract
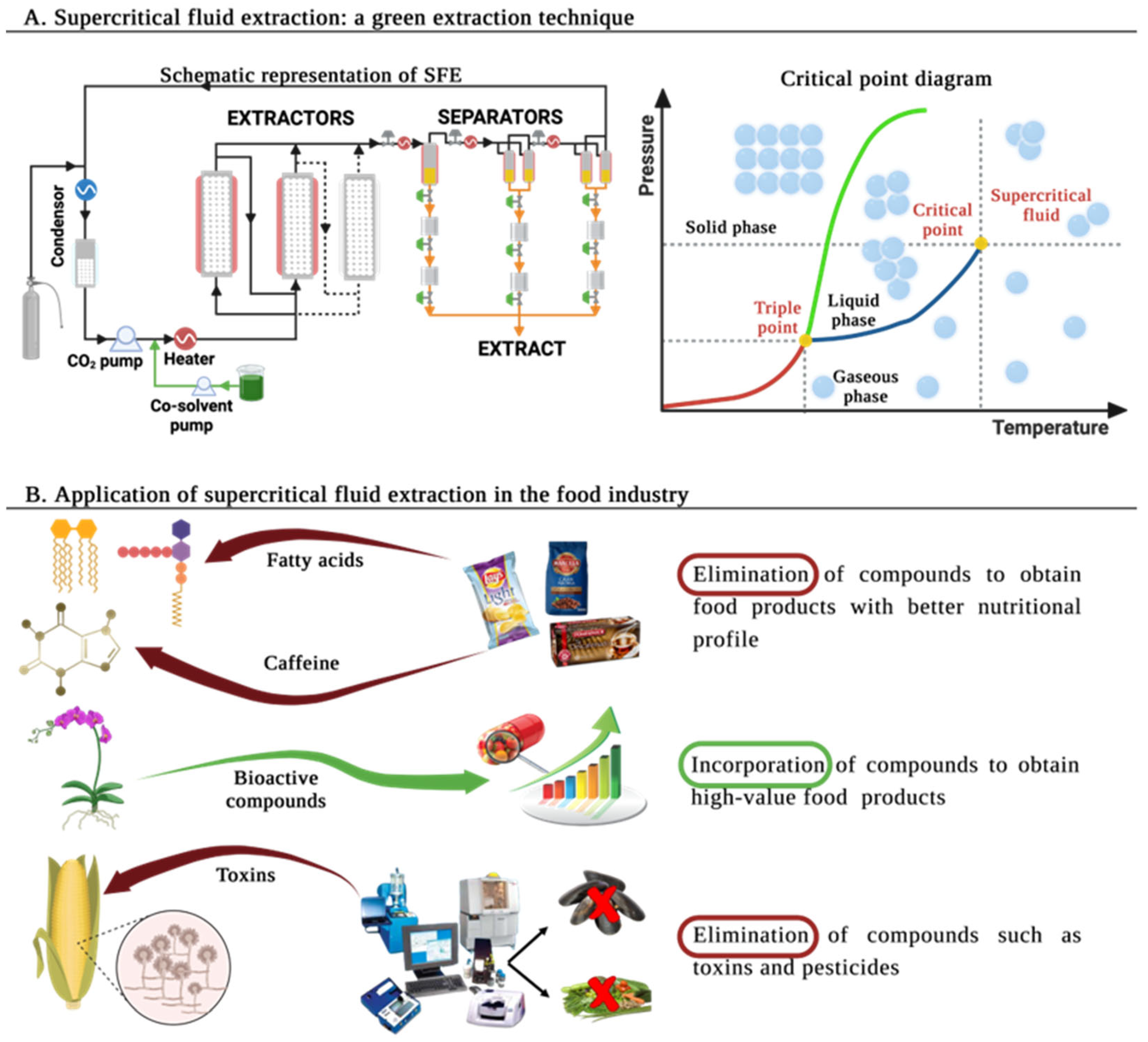
:1. Introducing Supercritical Fluid Extraction (SFE)
2. Supercritical Fluid Extraction (SFE) in the Food Industry
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Braga, M.E.M.; Gaspar, M.C.; de Sousa, H.C. Supercritical Fluid Technology for Agrifood Materials Processing. Curr. Opin. Food Sci. 2023, 50, 100983. [Google Scholar] [CrossRef]
- Ray, A.; Dubey, K.K.; Marathe, S.J.; Singhal, R. Supercritical Fluid Extraction of Bioactives from Fruit Waste and Its Therapeutic Potential. Food Biosci. 2023, 52, 102418. [Google Scholar] [CrossRef]
- Herrero, M.; Mendiola, J.A.; Cifuentes, A.; Ibáñez, E. Supercritical Fluid Extraction: Recent Advances and Applications. J. Chromatogr. A 2010, 1217, 2495–2511. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Liu, S.; Wang, H.; Zhang, M. Supercritical CO2 Fluid Extraction, Physicochemical Properties, Antioxidant Activities and Hypoglycemic Activity of Polysaccharides Derived from Fallen Ginkgo Leaves. Food Biosci. 2021, 42, 101153. [Google Scholar] [CrossRef]
- López-Hortas, L.; Rodríguez, P.; Díaz-Reinoso, B.; Gaspar, M.C.; de Sousa, H.C.; Braga, M.E.M.; Domínguez, H. Supercritical Fluid Extraction as a Suitable Technology to Recover Bioactive Compounds from Flowers. J. Supercrit. Fluids 2022, 188, 105652. [Google Scholar] [CrossRef]
- Song, L.; Liu, P.; Yan, Y.; Huang, Y.; Bai, B.; Hou, X.; Zhang, L. Supercritical CO2 Fluid Extraction of Flavonoid Compounds from Xinjiang Jujube (Ziziphus Jujuba Mill.) Leaves and Associated Biological Activities and Flavonoid Compositions. Ind. Crops Prod. 2019, 139, 111508. [Google Scholar] [CrossRef]
- Frohlich, P.C.; Santos, K.A.; Ascari, J.; Santos Refati, J.R.D.; Palú, F.; Cardozo-Filho, L.; da Silva, E.A. Antioxidant Compounds and Eugenol Quantification of Clove (Syzygium Aromaticum) Leaves Extracts Obtained by Pressurized Liquid Extraction and Supercritical Fluid Extraction. J. Supercrit. Fluids 2023, 196, 105865. [Google Scholar] [CrossRef]
- Santos, O.V.; Lorenzo, N.D.; Souza, A.L.G.; Costa, C.E.F.; Conceição, L.R.V.; Lannes, S.C.S.; Teixeira-Costa, B.E. CO2 Supercritical Fluid Extraction of Pulp and Nut Oils from Terminalia Catappa Fruits: Thermogravimetric Behavior, Spectroscopic and Fatty Acid Profiles. Food Res. Int. 2021, 139, 109814. [Google Scholar] [CrossRef] [PubMed]
- Pise, V.H.; Thorat, B.N. Supercritical Fluid Extraction of Dried Surangi Flowers (Mammea Suriga). Ind. Crops Prod. 2022, 186, 115268. [Google Scholar] [CrossRef]
- Wagner, M.E.; French, J.; Rizvi, S.S.H. Supercritical Fluid Extraction of Oil from Potato Chips: Two Scale Comparison and Mathematical Modeling. J. Food Eng. 2013, 118, 100–107. [Google Scholar] [CrossRef]
- De Marco, I.; Riemma, S.; Iannone, R. Life Cycle Assessment of Supercritical CO2 Extraction of Caffeine from Coffee Beans. J. Supercrit. Fluids 2018, 133, 393–400. [Google Scholar] [CrossRef]
- Shanakhat, H.; Sorrentino, A.; Raiola, A.; Romano, A.; Masi, P.; Cavella, S. Current Methods for Mycotoxins Analysis and Innovative Strategies for Their Reduction in Cereals: An Overview. J. Sci. Food Agric. 2018, 98, 4003–4013. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perez-Vazquez, A.; Barciela, P.; Carpena, M.; Donn, P.; Seyyedi-Mansour, S.; Cao, H.; Fraga-Corral, M.; Otero, P.; Simal-Gandara, J.; Prieto, M.A.; et al. Supercritical Fluid Extraction as a Potential Extraction Technique for the Food Industry. Eng. Proc. 2023, 37, 115. https://doi.org/10.3390/ECP2023-14674
Perez-Vazquez A, Barciela P, Carpena M, Donn P, Seyyedi-Mansour S, Cao H, Fraga-Corral M, Otero P, Simal-Gandara J, Prieto MA, et al. Supercritical Fluid Extraction as a Potential Extraction Technique for the Food Industry. Engineering Proceedings. 2023; 37(1):115. https://doi.org/10.3390/ECP2023-14674
Chicago/Turabian StylePerez-Vazquez, Ana, Paula Barciela, Maria Carpena, Pauline Donn, Sepidar Seyyedi-Mansour, Hui Cao, Maria Fraga-Corral, Paz Otero, Jesus Simal-Gandara, Mguel A. Prieto, and et al. 2023. "Supercritical Fluid Extraction as a Potential Extraction Technique for the Food Industry" Engineering Proceedings 37, no. 1: 115. https://doi.org/10.3390/ECP2023-14674
APA StylePerez-Vazquez, A., Barciela, P., Carpena, M., Donn, P., Seyyedi-Mansour, S., Cao, H., Fraga-Corral, M., Otero, P., Simal-Gandara, J., Prieto, M. A., & Cassani, L. (2023). Supercritical Fluid Extraction as a Potential Extraction Technique for the Food Industry. Engineering Proceedings, 37(1), 115. https://doi.org/10.3390/ECP2023-14674











