Dynamic Model to Expand Energy Storage in Form of Battery and Hydrogen Production Using Solar Powered Water Electrolysis for Off Grid Communities †
Abstract
:1. Introduction
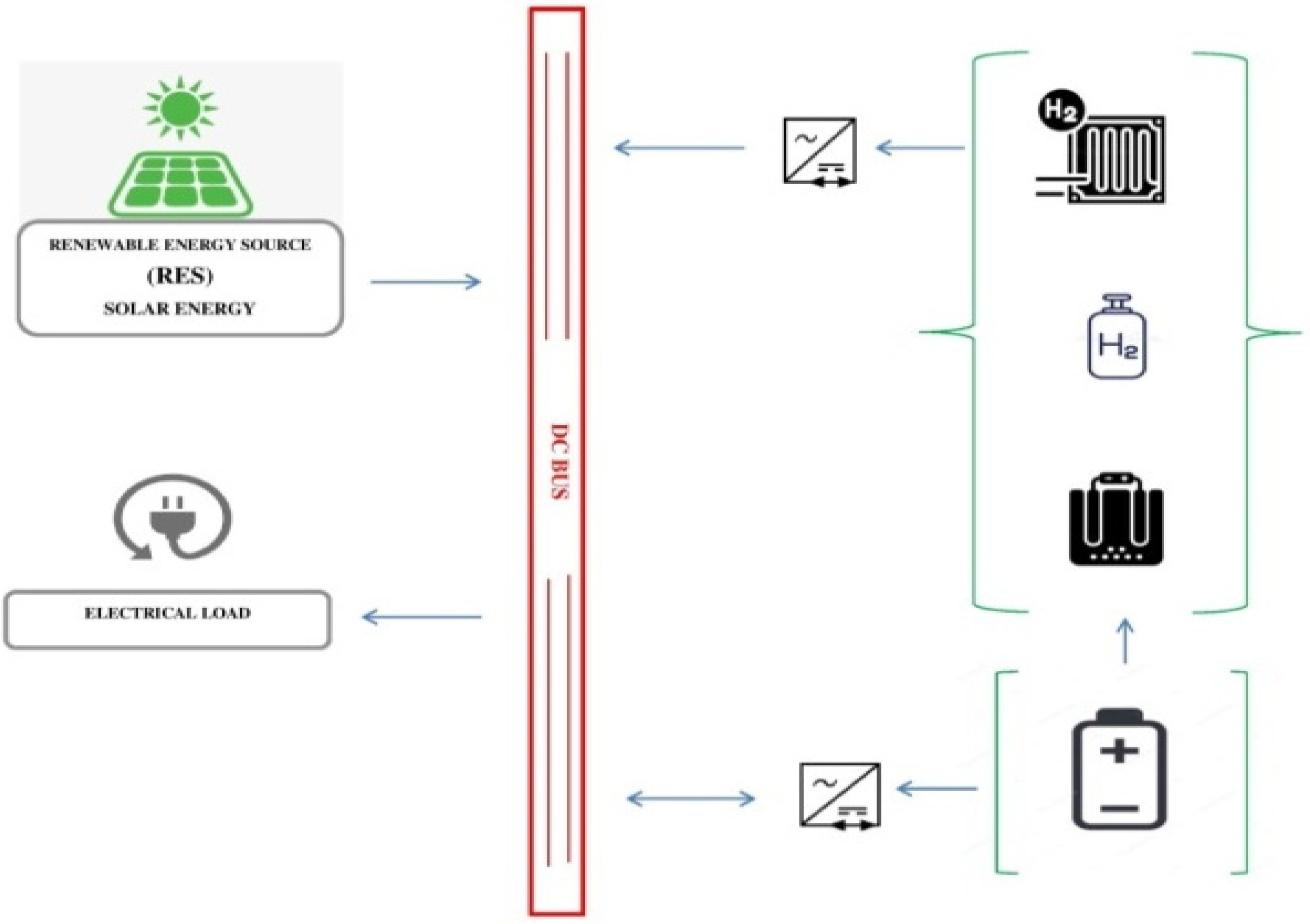
2. Methodology
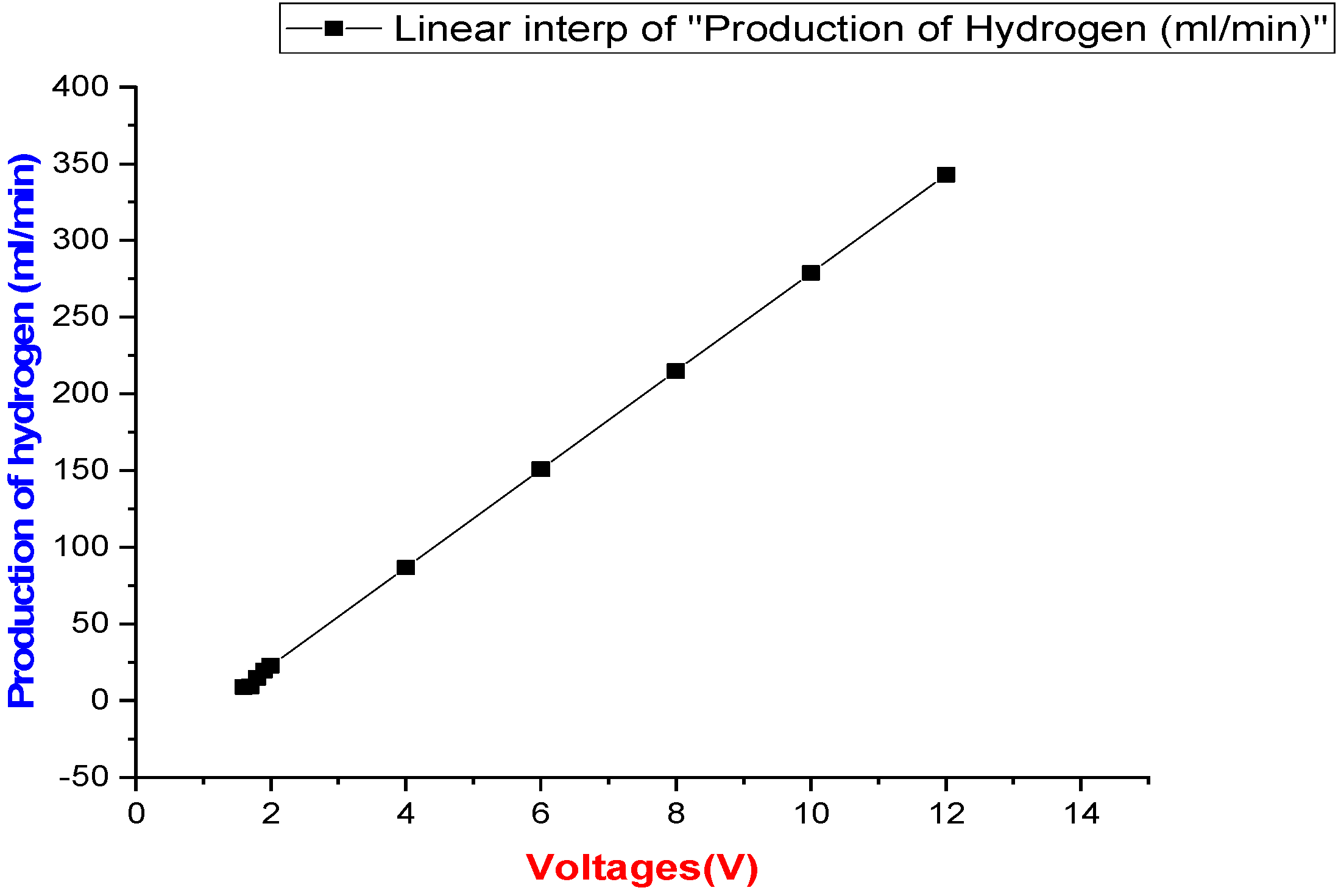
3. Results and Discussion
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Abdin, Z.; Webb, C.; Grey, M. Solar hydrogen hybrid energy systems for off-grid electricity supply: A critical review. Renew. Sustain. Energy Rev. 2015, 52, 1791–1808. [Google Scholar] [CrossRef]
- Höök, M.; Tang, X. Depletion of fossil fuels and anthropogenic climat change—A review. Energy Policy 2013, 52, 797–809. [Google Scholar] [CrossRef] [Green Version]
- Krieger, E.M.; Arnold, C.B. Effects of undercharge and internal loss on the rate dependence of battery charge storage efficiency. J. Power Sources 2012, 210, 286–291. [Google Scholar] [CrossRef]
- Bagheri, M.; Shirzadi, N.; Bazdar, E.; Kennedy, C.A. Optimal planning of hybrid renewable energy infrastructure for urban sustainability: Green Vancouver. Renew. Sustain. Energy Rev. 2018, 95, 254–264. [Google Scholar] [CrossRef]
- Barzola-Monteses, J.; Espinoza-Andaluz, M. Performance Analysis of Hybrid Solar/H2/Battery Renewable Energy System for Residential Electrification. Energy Procedia 2019, 158, 9–14. [Google Scholar] [CrossRef]
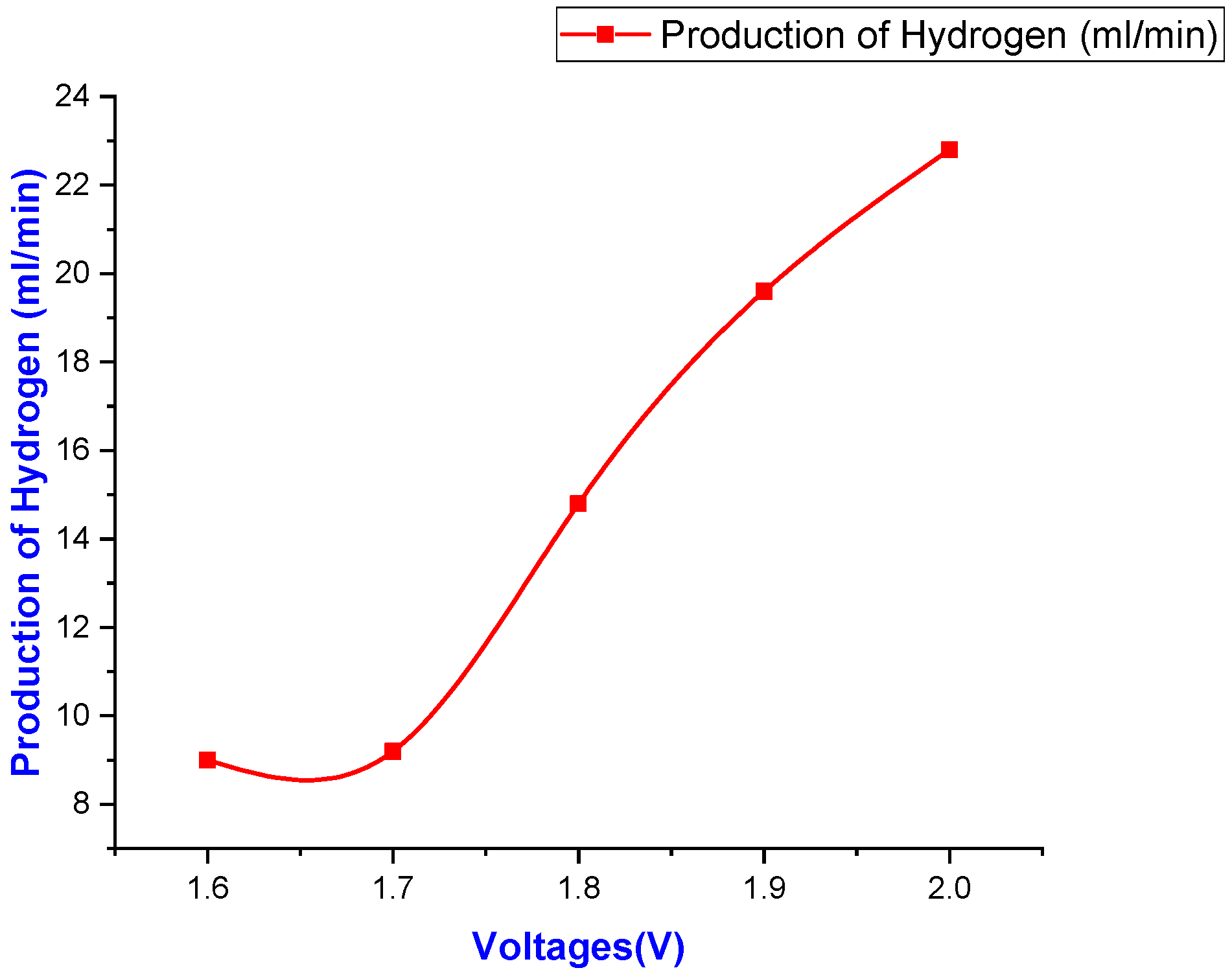
| Sr No. | Particulars | Practical Readings | ||||
|---|---|---|---|---|---|---|
| 1.6 V | 1.7 V | 1.8 V | 1.9 V | 2.0 V | ||
| 1st Reading | H2/(mL) | 13 | 12 | 18 | 24 | 24 |
| 2nd Reading | H2/(mL) | 8 | 10 | 14 | 18 | 27 |
| 3rd Reading | H2/(mL) | 8 | 8 | 14 | 20 | 24 |
| 4th Reading | H2/(mL) | 8 | 8 | 14 | 18 | 25 |
| 5th Reading | H2/(mL) | 8 | 8 | 14 | 18 | 23 |
| Average Reading | H2/(mL) | 9 | 9.2 | 14.8 | 19.6 | 24.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mushtaq, A.; Hussain, T.; Ayub, K.S.; Haider, M.S. Dynamic Model to Expand Energy Storage in Form of Battery and Hydrogen Production Using Solar Powered Water Electrolysis for Off Grid Communities. Eng. Proc. 2021, 12, 97. https://doi.org/10.3390/engproc2021012097
Mushtaq A, Hussain T, Ayub KS, Haider MS. Dynamic Model to Expand Energy Storage in Form of Battery and Hydrogen Production Using Solar Powered Water Electrolysis for Off Grid Communities. Engineering Proceedings. 2021; 12(1):97. https://doi.org/10.3390/engproc2021012097
Chicago/Turabian StyleMushtaq, Ali, Tajjamal Hussain, Khurram Shahzad Ayub, and Muhammad Salman Haider. 2021. "Dynamic Model to Expand Energy Storage in Form of Battery and Hydrogen Production Using Solar Powered Water Electrolysis for Off Grid Communities" Engineering Proceedings 12, no. 1: 97. https://doi.org/10.3390/engproc2021012097
APA StyleMushtaq, A., Hussain, T., Ayub, K. S., & Haider, M. S. (2021). Dynamic Model to Expand Energy Storage in Form of Battery and Hydrogen Production Using Solar Powered Water Electrolysis for Off Grid Communities. Engineering Proceedings, 12(1), 97. https://doi.org/10.3390/engproc2021012097





