1. Introduction
Phosphorus is an essential element required by living organisms [
1]. However, its use in various human activities (industrial, agricultural, and domestic) can lead to the contamination of aquatic systems, generating negative effects such as eutrophication of rivers, lakes, reservoirs, and estuaries. This causes algae proliferation and affects the quality of water and biodiversity in these ecosystems [
2,
3].
Chemical and biological processes for removing this substance from contaminated water have been developed [
4,
5]. One such process is adsorption, which has been evaluated using zeolites, bentonite, ion exchange resins, nanoparticles, and aluminum oxides as quick and effective methods for removing these contaminants [
2].
Specifically, biochar, a solid product formed by the thermal decomposition of biomass, has emerged as a low-cost alternative with a high surface area and carbon content that can be used to remove these substances [
2,
6].
Various materials have been evaluated as biomass sources for obtaining biochar to be used in removing phosphates from aqueous solutions, including eggshells, corn straw, peanuts, wheat straw,
Sacha Inchi cuticle, rice straw, palm fiber, and potato peel [
7,
8,
9,
10,
11,
12,
13,
14,
15].
However, many of these studies have been conducted only on a laboratory scale. Therefore, this study evaluated the removal of phosphates using biochar obtained from Sacha Inchi husks and eggshells. This was performed using a pilot-scale filtration system to assess the performance of the process at this scale.
2. Materials and Methods
2.1. Sacha Inchi
The cuticles of
Sacha Inchi seeds were obtained from the Agroindustrial Plant of the Universidad de La Salle, located at the Utopía Campus in Yopal, Casanare, Colombia (coordinates 5°19′23.1″ N 72°17′31.3″ W,
Figure 1). This cuticle was obtained after the dehulling of the
Sacha Inchi seed prior to the oil extraction process.
2.2. Eggshell
The eggshells used in this study were collected from a commercial food processing facility in Bogotá, Colombia. After arrival at the laboratory, the material was washed with distilled water to remove the membrane and any type of contaminant adhered to its surface.
2.3. Bioadsorbent Preparation
Eggshell and
Sacha Inchi cuticle were mixed in a 1:10 ratio and subjected to grinding and pyrolysis. Pyrolysis was carried out at 400 °C for 65 min (15 min preheating, 20 min at set temperature, and 30 min cooling). The obtained material was transferred to a desiccator (Simax®, Sázava, Czech Republic ) and sieved, retaining the fraction with a particle size between 0.150 and 0.420 mm [
12].
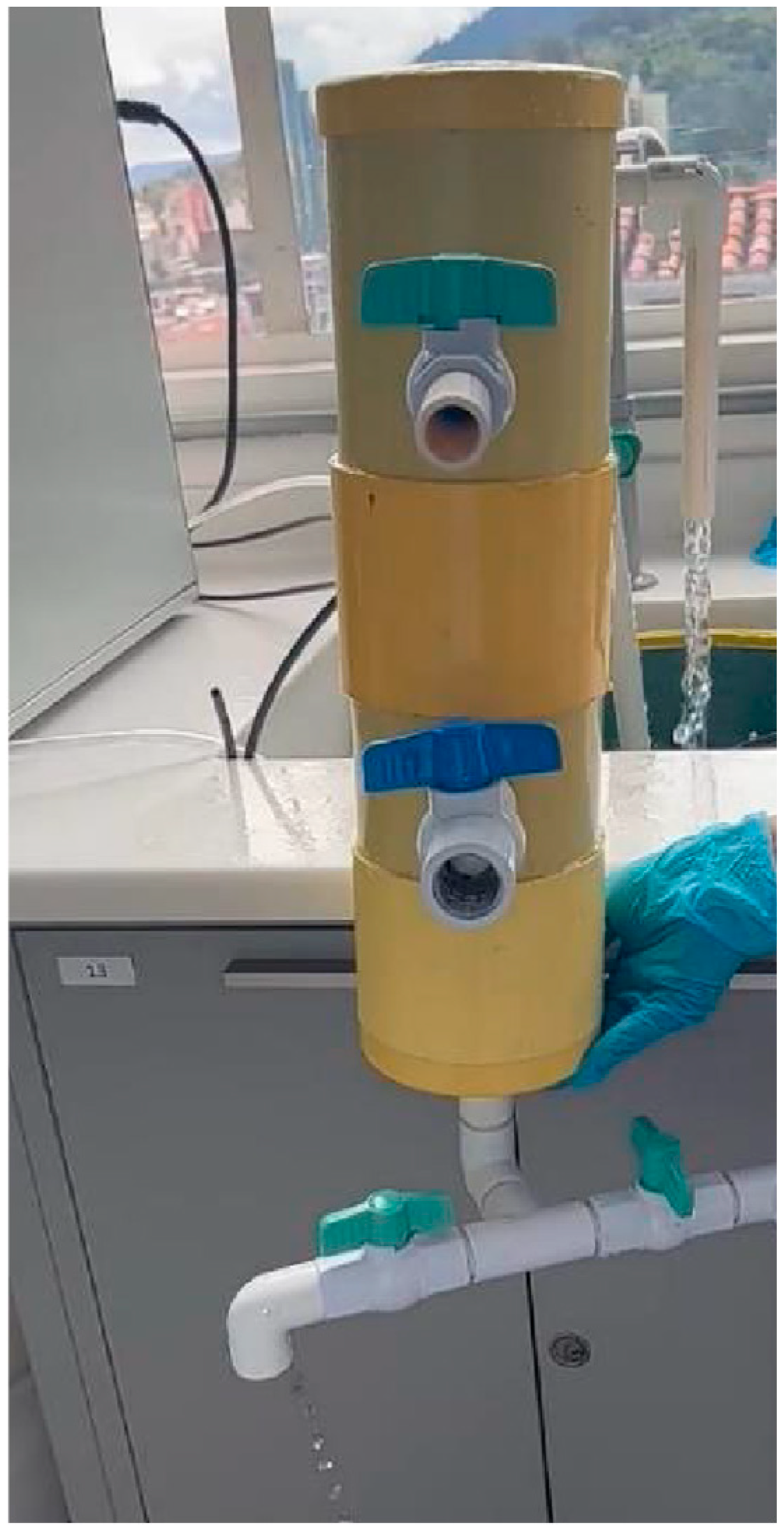
2.4. Filtration System
For the filter design, it was established that a backwash system would be implemented using a flow rate of 0.020 m
3/h with a filter media height of 0.16 m and a filtration rate of 2.52 m
3/(m
2·h), equivalent to 60.48 m
3/(m
2·d). To simplify the construction of the filter, a diameter of 0.10 m was established, which is a size that could be found in a 4-inch sanitary PVC pipe. The characteristics of the filtration system are listed in
Table 1.
Through the pilot filter, synthetic water was prepared with a concentration of 133 mg/L of PO
4 −3 (NaH
2PO
4·H
2O, Merck, Darmstadt, Germany) to conduct a single test. This concentration was established based on the literature, according to the phosphate concentration of groundwater and surface water in real phosphate-contaminated sites [
16]. For the pilot test, 8 L of the corresponding concentrations were prepared (a temperature of 25 °C and pH: 5.0 were kept constant during the tests). Five liters leaked through the constructed filter because the pump used to drive the water required an additional three liters to remain submerged and prevent damage. Three samples were collected at three different moments during the test: one when 1 L had been filtered, the second when 2.5 L had been filtered, and the last when 5 L had been filtered. The samples were analyzed using spectrophotometry to determine phosphate adsorption (phosphate content was determined using the ascorbic acid method with a HACH DR 3800 spectrophotometer and PhosVer® 3 reactive, HACH, Loveland, CO, USA) [
12]. The test was performed in duplicate. Backwashing was not performed because the focus of the analysis was on phosphate adsorption during the filtration process.
3. Results and Discussion
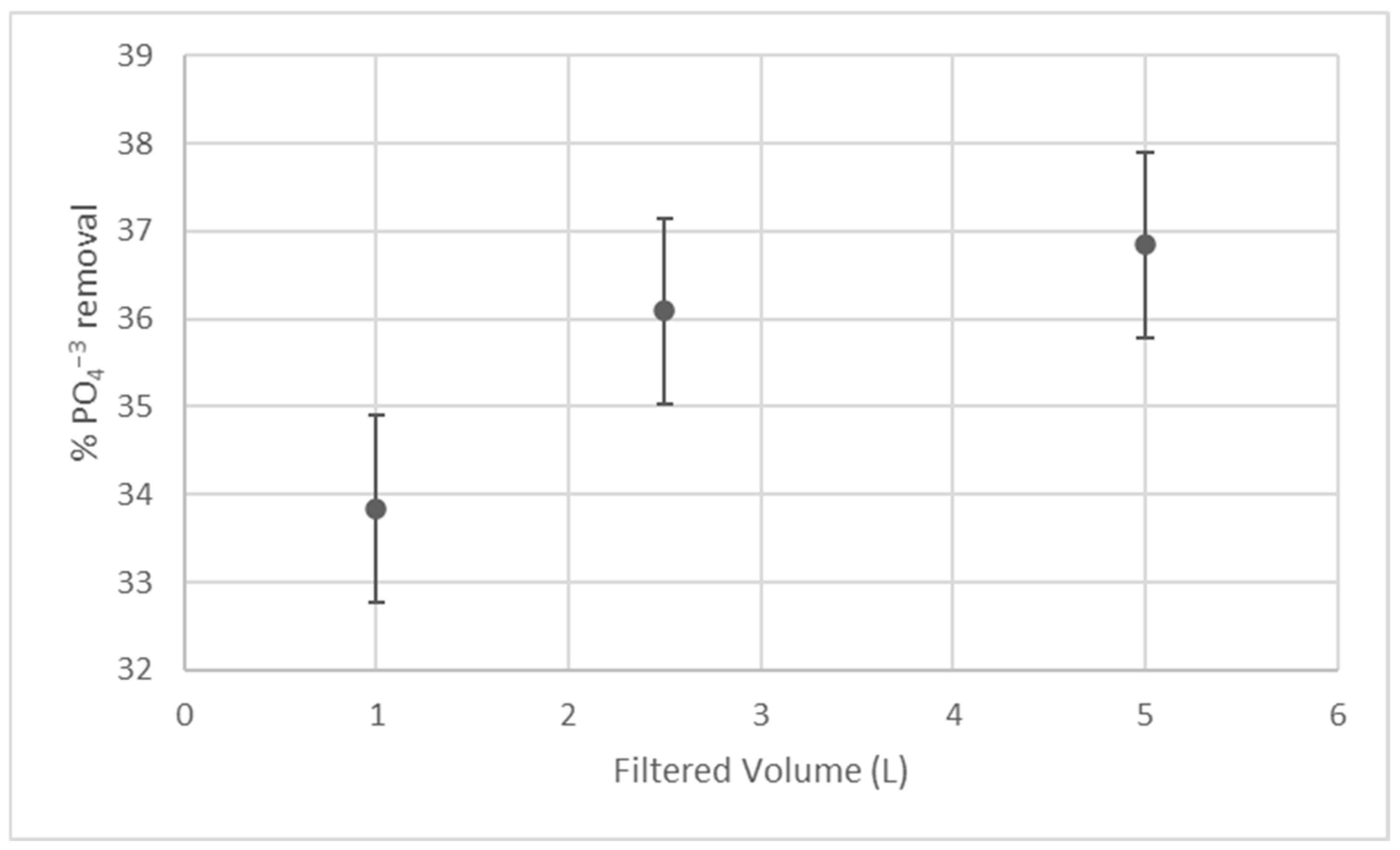
Figure 2 summarizes the results of phosphate removal using the developed pilot equipment.
As illustrated in
Figure 2, the progression of phosphate removal with increasing filtered volume demonstrates the ongoing interaction between the contaminated solution and the active sites available on the bioadsorbent. During the initial phase of filtration (first liter), a higher removal efficiency is observed due to the abundance of calcium-rich active sites on the surface of the eggshell and
Sacha Inchi bioadsorbent, facilitating rapid adsorption and precipitation of phosphate. As filtration continues and additional volumes of contaminated water are processed, these reactive sites become increasingly occupied or partially saturated, leading to a gradual decline or stabilization in removal efficiency as the filtered volume increases. This behavior is typical of fixed-bed filtration systems operating in continuous flow, where mass transfer limitations and the reduced availability of active sites influence performance over time. The observed trend suggests that phosphate removal is governed by the availability of surface sites rather than hydraulic instability, confirming that the system maintains a constant but finite adsorption capacity during single-pass operation.
As a result, an average PO
4−3 removal percentage of 35.58 ± 1.57% was obtained. It is relevant to note that the filtration time required to recover 5 L of filtrate was slightly more than 12 min. This result was compared with the results reported in a similar laboratory-scale study, where a solution with a PO
4−3 concentration of 300 mg/L was used, with a ratio of 0.1 g of adsorbent per 100 mL of solution, at 25 °C, pH 5, and 150 rpm, achieving a phosphate removal of 28.44% [
12]. It was established that the evaluated system maintained its level of removal and could even improve when operated at the pilot scale.
In contrast, the presence of calcium in the biosorbent materials, particularly in eggshells, is essential for phosphate removal. Calcium carbonate (CaCO
3) releases Ca
2+ ions, which react with phosphate ions to form calcium phosphate compounds [
16,
17]. This mechanism facilitates effective yet moderate removal, constrained by the uneven distribution of calcium and the limited number of available active sites. Overall, the findings confirm that eggshells combined with
Sacha Inchi cuticles possess significant potential as natural biosorbent materials.
To enhance system performance, it is recommended to apply controlled pyrolysis treatment to the materials to increase their porosity and the number of available active sites for adsorption [
18]. It is possible to consider the application of graphene and its derivatives to increase the potential of the evaluated adsorbent, taking advantage of functionalization of graphene-based adsorbents, as well as their adsorption performance and mechanism of action in the removal of inorganic and organic pollutants [
19,
20]. Additionally, incorporating a backwashing system in the pilot filter would enable the regeneration of the filtering medium, prevent early saturation, and extend the system’s lifespan, thereby promoting a more efficient and sustainable treatment process.
The study identified that the efficiency of phosphate removal was constrained by a brief hydraulic retention time of 10 min under continuous flow conditions. This limitation resulted in reduced interaction between phosphate ions and calcium-rich surfaces compared to discontinuous systems at equilibrium. Additionally, the pH was not adjusted to promote calcium-phosphate precipitation. Furthermore, the filtration process was conducted in a single-pass mode without backwashing or regeneration of the medium, leading to the progressive saturation of the reactive sites.
4. Conclusions
This study presents a novel approach by developing and evaluating a hybrid bioadsorbent synthesized from eggshells and Sacha Inchi seed cuticles. These two agro-industrial residues have not been commonly combined for the purpose of phosphate removal. In contrast to previous research, which predominantly focused on batch-scale experiments, this investigation assesses the material within a pilot-scale filtration system under realistic hydraulic conditions. Additionally, the utilization of locally available waste materials and phosphate concentrations that are representative of those found in contaminated water bodies underscores the practicality and sustainability of the proposed treatment system.
In conclusion, although the removal percentage is lower than that reported by other authors using similar materials as adsorbents, the difference is likely due to the conditions associated with the preparation of the adsorbent. Other conditions could be assessed to improve the removal percentage, contributing to the understanding of these residues and their valorization for the development of sustainable processes.
The tests conducted both in the laboratory and in the pilot filter showed similar removal percentages, with an average value of 36.84%, demonstrating the reproducibility and stability of the system when scaled up. This behavior indicates that the material’s efficiency is maintained when moving from an experimental to a practical scale, validating its potential for use in wastewater treatment systems.
The results obtained from the pilot filter confirmed the effectiveness of the mixed biosorbent system in reducing phosphate concentrations under real operating conditions. The stability of the removal percentage and sustained adsorption capacity showed that continuous flow did not negatively affect the performance, reinforcing the technical feasibility of this process for large-scale applications.
The filtration system assessed in this study is distinguished by its low capital and operational costs, as it is constructed from readily available materials such as PVC piping and employs a bioadsorbent derived from agroindustrial waste, specifically eggshells and Sacha Inchi cuticles, which incur negligible or no acquisition costs. The primary expenses associated with the system pertain to basic preprocessing steps, including washing, grinding, and pyrolysis, as well as simple pumping requirements. Consequently, the total system cost is substantially lower than that of conventional physicochemical phosphate removal technologies. Regarding the product obtained—partially purified water with a phosphate removal efficiency of approximately 36%—the system offers a high value-to-cost ratio, particularly for applications in decentralized or rural settings where low-cost treatment solutions are essential. Therefore, the proposed system represents an economically attractive and sustainable alternative for preliminary phosphate removal and water quality enhancement.
Author Contributions
Conceptualization, A.C.R.-T., N.L.L.-B., C.P.-G. and A.M.O.-Á.; methodology, A.C.R.-T., N.L.L.-B., C.P.-G. and A.M.O.-Á.; formal analysis A.C.R.-T., N.L.L.-B., C.P.-G. and A.M.O.-Á.; investigation, A.C.R.-T. and N.L.L.-B.; writing—original draft preparation, A.C.R.-T., N.L.L.-B., C.P.-G. and A.M.O.-Á.; writing—review and editing, A.C.R.-T., N.L.L.-B., C.P.-G. and A.M.O.-Á.; funding acquisition, A.M.O.-Á. All authors have read and agreed to the published version of the manuscript.
Funding
This work was carried out within the framework of the project: “Opportunities for Technological Development in The Processing of Sacha Inchi, Produced in Utopia (Yopal-Casanare)”, code IALI212-190, financed by the Vice-Rectorate for Research and Transfer (Vrit) of the University of La Salle, Bogotá.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tiessen, H. Phosphorus in the Global Environment. In The Ecophysiology of Plant-Phosphorus Interactions; White, P.J., Hammond, J.P., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2008; pp. 1–7. ISBN 978-1-4020-8435-5. [Google Scholar]
- Zhang, M.; Song, G.; Gelardi, D.L.; Huang, L.; Khan, E.; Mašek, O.; Parikh, S.J.; Ok, Y.S. Evaluating Biochar and Its Modifications for the Removal of Ammonium, Nitrate, and Phosphate in Water. Water Res. 2020, 186, 116303. [Google Scholar] [CrossRef] [PubMed]
- Razanajatovo, M.R.; Gao, W.; Song, Y.; Zhao, X.; Sun, Q.; Zhang, Q. Selective Adsorption of Phosphate in Water Using Lanthanum-Based Nanomaterials: A Critical Review. Chin. Chem. Lett. 2021, 32, 2637–2647. [Google Scholar] [CrossRef]
- Velusamy, K.; Periyasamy, S.; Kumar, P.S.; Vo, D.-V.N.; Sindhu, J.; Sneka, D.; Subhashini, B. Advanced Techniques to Remove Phosphates and Nitrates from Waters: A Review. Environ. Chem. Lett. 2021, 19, 3165–3180. [Google Scholar] [CrossRef]
- Ramasahayam, S.K.; Guzman, L.; Gunawan, G.; Viswanathan, T. A Comprehensive Review of Phosphorus Removal Technologies and Processes. J. Macromol. Sci. Part A 2014, 51, 538–545. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Kim, S.; Igalavithana, A.D.; Hashimoto, Y.; Choi, Y.-E.; Mukhopadhyay, R.; Sarkar, B.; Ok, Y.S. Fe(III) Loaded Chitosan-Biochar Composite Fibers for the Removal of Phosphate from Water. J. Hazard. Mater. 2021, 415, 125464. [Google Scholar] [CrossRef]
- Bus, A.; Budzanowska, K.; Karczmarczyk, A.; Baryła, A. Raw and Calcined Eggshells as P-Reactive Materials in a Circular Economy Approach. Sustainability 2025, 17, 1191. [Google Scholar] [CrossRef]
- Liu, X.; Lv, J. Efficient Phosphate Removal from Wastewater by Ca-Laden Biochar Composites Prepared from Eggshell and Peanut Shells: A Comparison of Methods. Sustainability 2023, 15, 1778. [Google Scholar] [CrossRef]
- Sun, C.; Huang, C.; Wang, P.; Yin, J.; Tian, H.; Liu, Z.; Xu, H.; Zhu, J.; Hu, X.; Liu, Z. Low-Cost Eggshell-Fly Ash Adsorbent for Phosphate Recovery: A Potential Slow-Release Phosphate Fertilizer. Water Res. 2024, 255, 121483. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Li, C.; Sumita; Pang, W. Study on the Removal Efficacy and Mechanism of Phosphorus from Wastewater by Eggshell-Modified Biochar. Water Environ. Res. 2024, 96, e10998. [Google Scholar] [CrossRef] [PubMed]
- Steiger, B.G.K.; Bui, N.T.; Babalola, B.M.; Wilson, L.D. Eggshell Incorporated Agro-Waste Adsorbent Pellets for Sustainable Orthophosphate Capture from Aqueous Media. RSC Sustain. 2024, 2, 1498–1507. [Google Scholar] [CrossRef]
- López-Bermúdez, N.L.; Rodríguez-Torres, A.C.; Otálvaro-Álvarez, Á.M.; Peña-Guzmán, C.A. Phosphate Adsorption from Aqueous Solutions Using Eggshell and Sacha Inchi (Plukenetia volubilis) Mixture. Civ. Eng. J. 2025, 11, 2918–2932. [Google Scholar] [CrossRef]
- Liu, X.; Shen, F.; Qi, X. Adsorption Recovery of Phosphate from Aqueous Solution by CaO-Biochar Composites Prepared from Eggshell and Rice Straw. Sci. Total Environ. 2019, 666, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Quisperima, A.; Pérez, S.; Flórez, E.; Acelas, N. Valorization of Potato Peels and Eggshells Wastes: Ca-Biocomposite to Remove and Recover Phosphorus from Domestic Wastewater. Bioresour. Technol. 2022, 343, 126106. [Google Scholar] [CrossRef] [PubMed]
- Pérez, S.; Muñoz-Saldaña, J.; Garcia-Nunez, J.A.; Acelas, N.; Flórez, E. Unraveling the Ca–P Species Produced over the Time during Phosphorus Removal from Aqueous Solution Using Biocomposite of Eggshell-Palm Mesocarp Fiber. Chemosphere 2022, 287, 132333. [Google Scholar] [CrossRef] [PubMed]
- Ou, W.; Lan, X.; Guo, J.; Cai, A.; Liu, P.; Liu, N.; Liu, Y.; Lei, Y. Preparation of Iron/Calcium-Modified Biochar for Phosphate Removal from Industrial Wastewater. J. Clean. Prod. 2023, 383, 135468. [Google Scholar] [CrossRef]
- Cao, L.; Ouyang, Z.; Chen, T.; Huang, H.; Zhang, M.; Tai, Z.; Long, K.; Sun, C.; Wang, B. Phosphate Removal from Aqueous Solution Using Calcium-Rich Biochar Prepared by the Pyrolysis of Crab Shells. Environ. Sci. Pollut. Res. 2022, 29, 89570–89584. [Google Scholar] [CrossRef] [PubMed]
- Pap, S.; Karmann, C.; Thompson, T.; McConnell, R.; Kennedy, T.; Taggart, M.A. Insights into Phosphate Removal and Recovery from Wastewater Using Biosolids Biochar: Pyrolysis Optimisation, Mechanistic and Column Studies. J. Water Process Eng. 2025, 75, 107954. [Google Scholar] [CrossRef]
- Verma, S.; Nadagouda, M.N. Graphene-Based Composites for Phosphate Removal. ACS Omega 2021, 6, 4119–4125. [Google Scholar] [CrossRef] [PubMed]
- Laghlimi, C.; Moutcine, A.; Chtaini, A.; Isaad, J.; Soufi, A.; Ziat, Y.; Amhamdi, H.; Belkhanchi, H. Recent Advances in Electrochemical Sensors and Biosensors for Monitoring Drugs and Metabolites in Pharmaceutical and Biological Samples. ADMET DMPK 2023, 11, 151–173. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |