Seed Priming with Pectic-Oligosaccharides Improved Seed Germination and Growth of Chili †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of POS
2.3. Imbibition Curve
2.4. Seed Priming Treatment
2.5. Determinations of Seed Germination and Seedling Characteristics
2.6. Statistical Analyses
3. Results and Discussion
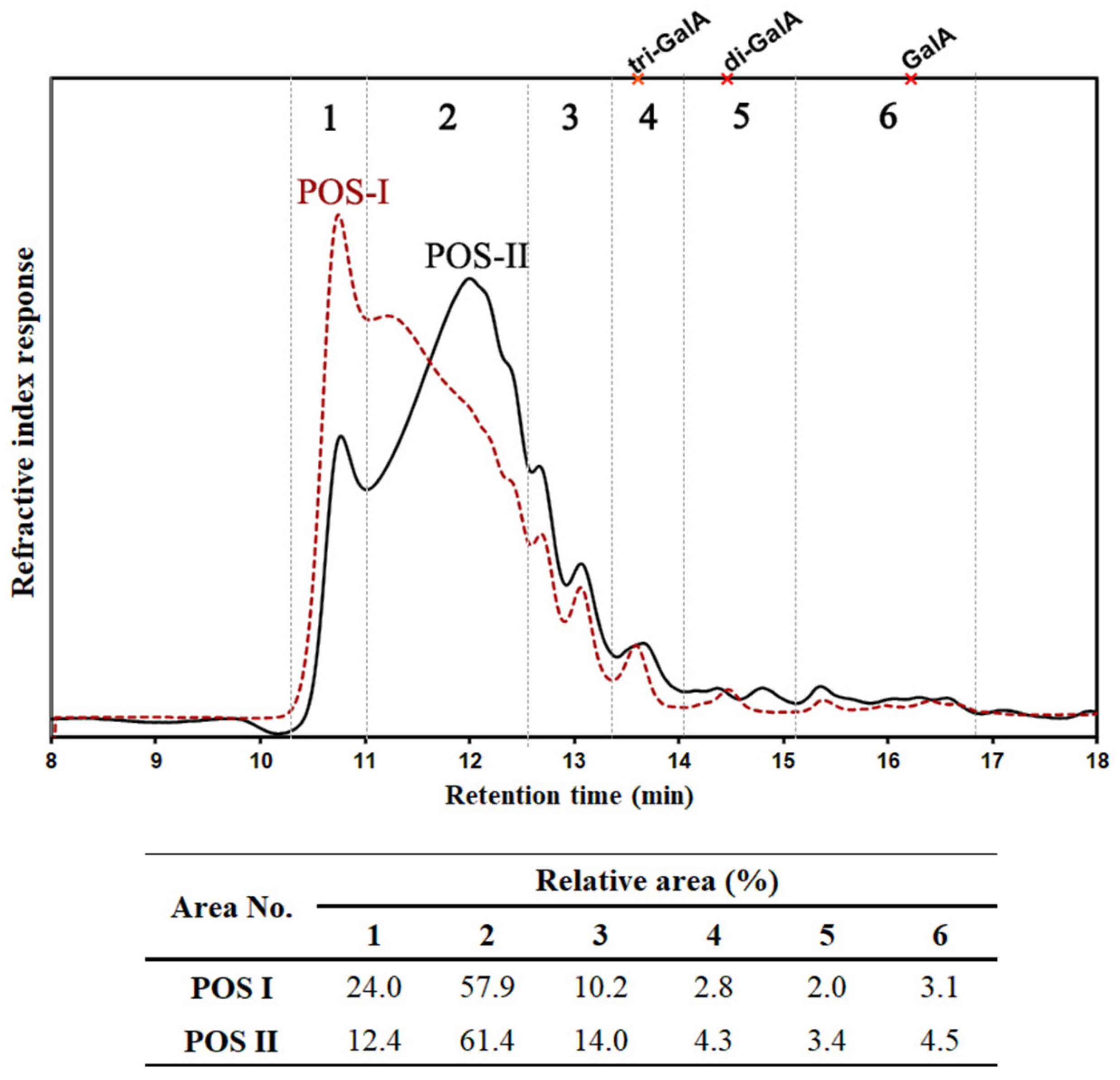
3.1. Size Distribution of POS
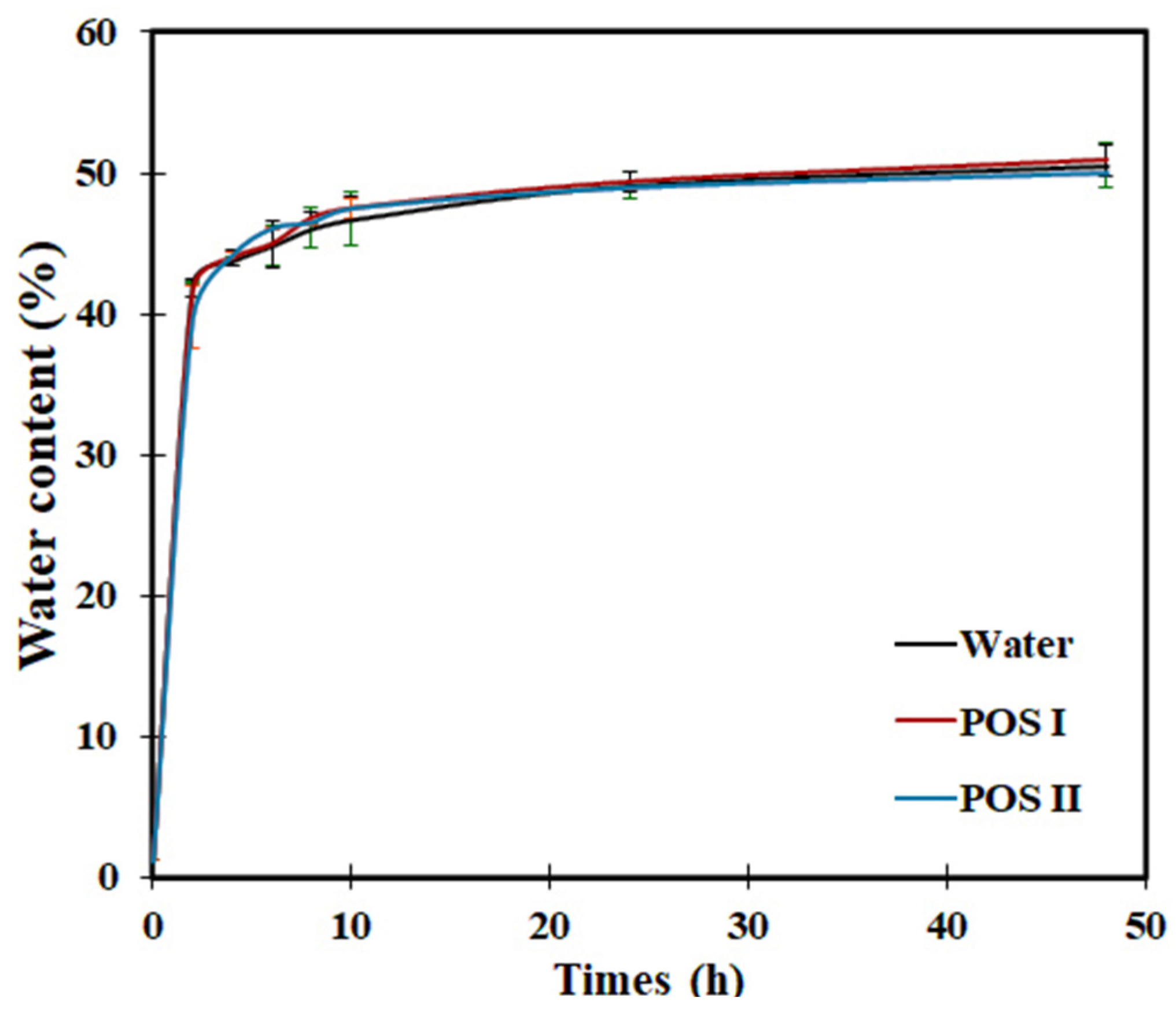
3.2. Imbibition Curve
3.3. Germination and Seed Growth Parameters
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Taylor, A.G.; Allen, P.S.; Bennett, M.A.; Bradford, K.J.; Burris, J.S.; Misra, M.K. Seed enhancements. Seed Sci. Res. 1998, 8, 245–256. [Google Scholar] [CrossRef]
- Lutts, S.; Benincasa, P.; Wojtyla, L.; Kubala, S.S.; Pace, R.; Lechowska, K.; Quinet, M.; Garnczarska, M. Seed Priming: New Comprehensive Approaches for an Old Empirical Technique. In New Challenges in Seed Biology—Basic and Translational Research Driving Seed Technology; Araujo, S., Balestrazzi, A., Eds.; IntechOpen: London, UK, 2016. [Google Scholar]
- Maiti, R.; Rajkumar, D.; Jagan, M.; Pramanik, K.; Vidyasagar, P. Effect of Seed Priming on Seedling Vigour and Yield of Tomato and Chilli. Int. J. Bio-Resour. Stress Manag. 2013, 4, 119–125. [Google Scholar]
- Thakur, M.; Sohal, B.S. Role of Elicitors in Inducing Resistance in Plants against Pathogen Infection: A Review. ISRN Biochem. 2013, 2013, 762412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Posmyk, M.M.; Szafranska, K. Biostimulators: A New Trend towards Solving an Old Problem. Front. Plant Sci. 2016, 7, 748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, X.; Jiang, X.; Hwang, H.; Liu, S.; Guan, H. Promotive effects of alginate-derived oligosaccharide on maize seed germination. J. Appl. Phycol. 2004, 16, 73–76. [Google Scholar] [CrossRef]
- Colman, S.L.; Salcedo, M.F.; Mansilla, A.Y.; Iglesiasa, M.J.; Fiol, D.F.; Saldañac, S.M.; Chevalier, A.A.; Casalongué, C.A.; Alvarez, A.A. Chitosan microparticles improve tomato seedling biomass and modulate hormonal, redox and defense pathways. Plant Physiol. Biochem. 2019, 143, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Nandhini, D.U.; Somasundaram, E. Lipo-Chito Oligosaccharides Enhances Germination Tolerance of Maize to Salinity Stress. Int. J. Curr. Microbiol. App. Sci. 2017, 6, 437–443. [Google Scholar] [CrossRef] [Green Version]
- Babbar, N.; Dejonghe, W.; Sforza, S.; Elst, K. Enzymatic pectic oligosaccharides (POS) production from sugar beet pulp using response surface methodology. J. Food Sci. 2017, 54, 3707–3715. [Google Scholar] [CrossRef] [PubMed]
- Beatriz, M.; Belén, G.; Patricia, G.; Beatriz, G.; Alonso, J.L. Pectic Oligosaccharides and Other Emerging Prebiotics. In Probiotics and Prebiotics in Human Nutrition and Health; Rao, V., Ed.; IntechOpen: London, UK, 2016. [Google Scholar]
- Gullón, B.; Gómez, B.; Martínez-Sabajanes, M.; Yánez, R.; Parajó, J.C.; Alonso, J.L. Pectic oligosaccharides: Manufacture and functional properties. Trends Food Sci. Technol. 2013, 30, 153–161. [Google Scholar] [CrossRef]
- Cabrera, J.C.; Wégria, G.; Onderwater, R.C.A.; González, G.; Nápoles, M.C.; Falcón-Rodríguez, A.B.; Costales, D.; Rogers, H.J.; Diosdado, E.; González, S.; et al. Practical use of oligosaccharins in agriculture. Acta Hortic. 2013, 1009, 195–212. [Google Scholar] [CrossRef]
- Wandee, Y.; Uttapap, D.; Mischnick, P.; Rungsardthong, V. Production of pectic-oligosaccharides from pomelo peel pectin by oxidative degradation with hydrogen peroxide. Food Chem. 2021, 348, 129078. [Google Scholar] [CrossRef] [PubMed]
- Association of Official Seed Analysts (AOSA). Rules for testing seeds. J. Seed Technol. 1990, 12, 1–112. [Google Scholar]
- Amnuaysin, N.; Korakotchakorn, H.; Chittapun, S.; Poolyarat, N. Seed germination and seedling growth of rice in response to atmospheric air dielectric-barrier discharge plasma. Songklanakarin J. Sci. Technol. 2018, 40, 819–823. [Google Scholar]
- Bewley, J.D.; Black, M. Seed: Physiology of Development and Germination, 2nd ed.; Springer: New York, NY, USA, 1994; pp. 1–445. [Google Scholar]
- Qi, F.; Zhang, F. Cell Cycle Regulation in the Plant Response to Stress. Front. Plant Sci. 2019, 10, 1765. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Treatments | Root Length (mm) | Shoot Length (mm) | Seedling Length (mm) | Vigor Index |
|---|---|---|---|---|
| Non-priming (Control) | 11.2 BC | 11.6 B | 22.9 B | 2062.0 C |
| Priming at 30 °C | ||||
| Water | 14.1 A | 15.7 A | 29.8 A | 2322.0 BC |
| POS-I | 13.3 AB | 17.7 A | 31.0 A | 2914.0 A |
| POS-II | 11.2 BC | 18.1 A | 29.3 A | 2693.9 AB |
| Priming at 50 °C | ||||
| Water | 6.0 E | 6.2 C | 12.2 D | 720.0 E |
| POS-I | 7.4 DE | 6.8 C | 14.2 CD | 925.7 DE |
| POS-II | 9.6 CD | 9.4 BC | 19.0 BD | 1178.0 D |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Udchumpisai, W.; Wandee, Y.; Kotatha, D.; Uttapap, D. Seed Priming with Pectic-Oligosaccharides Improved Seed Germination and Growth of Chili. Eng. Proc. 2021, 11, 41. https://doi.org/10.3390/ASEC2021-11159
Udchumpisai W, Wandee Y, Kotatha D, Uttapap D. Seed Priming with Pectic-Oligosaccharides Improved Seed Germination and Growth of Chili. Engineering Proceedings. 2021; 11(1):41. https://doi.org/10.3390/ASEC2021-11159
Chicago/Turabian StyleUdchumpisai, Wascharin, Yuree Wandee, Ditpon Kotatha, and Dudsadee Uttapap. 2021. "Seed Priming with Pectic-Oligosaccharides Improved Seed Germination and Growth of Chili" Engineering Proceedings 11, no. 1: 41. https://doi.org/10.3390/ASEC2021-11159
APA StyleUdchumpisai, W., Wandee, Y., Kotatha, D., & Uttapap, D. (2021). Seed Priming with Pectic-Oligosaccharides Improved Seed Germination and Growth of Chili. Engineering Proceedings, 11(1), 41. https://doi.org/10.3390/ASEC2021-11159






