1. Introduction
Dye-sensitized solar cells (DSSCs) have emerged as a promising alternative to conventional silicon-based photovoltaics due to their cost-effectiveness, ease of fabrication, and relatively high-power conversion efficiency under low-light conditions [
1,
2,
3]. A typical DSSC consists of a photoanode, a dye sensitizer, an electrolyte, and a counter electrode [
4]. The photoanode plays a critical role in dye adsorption, electron transport, and charge separation, directly influencing the overall device performance [
5]. Titanium dioxide (TiO
2) is widely used as a photoanode material in DSSCs due to its excellent chemical stability, appropriate bandgap (~3.2 eV for anatase), strong light scattering ability, and high surface area that facilitates dye molecule adsorption [
6]. Several approaches have been employed to fabricate TiO
2 films for DSSC applications, including sol–gel synthesis [
7], hydrothermal treatment [
8], electrophoretic deposition [
9], screen printing [
10], and magnetron sputtering [
11]. Among them, the sol–gel method is particularly attractive due to its low-cost processing, excellent compositional control, and its ability to produce uniform thin films at relatively low processing temperatures [
12]. However, pure sol–gel-derived TiO
2 films often suffer from limited charge transport properties and insufficient dye adsorption, leading to lower photovoltaic efficiency [
13,
14].
To address these limitations, various studies have been proposed, including the incorporation of TiO
2 nanoparticles into the sol–gel matrix [
15,
16]. Incorporating nanoparticles enhances porosity, increases the surface area for dye adsorption, and improves light harvesting through increased scattering. Additionally, nanocrystalline domains promote more efficient electron transport and reduce recombination losses due to shortened diffusion paths [
17]. Integrating TiO
2 nanoparticles of controlled size significantly enhances the device’s
Jsc and overall efficiency [
18].
In this study, TiO2 thin films were fabricated using the sol–gel method with and without the incorporation of 25 nm TiO2 nanoparticles. The films were deposited on fluorine-doped tin oxide (FTO) glass substrates using spin coating and annealed at 600 °C to achieve crystallization. The resulting electrodes were evaluated for their structural, morphological, optical, and photovoltaic properties. We investigated the effects of TiO2 nanoparticle incorporation on DSSC performance and proposed an optimized photoanode structure for enhanced solar energy conversion.
2. Experimental Method
The TiO2 sol–gel precursor was synthesized using titanium (IV) isopropoxide (TTIP, 97%, Sigma-Aldrich, St. Louis, MO, USA) as the starting material, dissolved in ethanol (99.8%, Merck, Darmstadt, Germany) with acetic acid (99.7%, Sigma-Aldrich) as a stabilizer. Deionized water was added to initiate hydrolysis, and the mixture was magnetically stirred (IKA RH digital stirrer, 500 rpm) for 2 h to form a homogeneous sol. For the nanoparticle-incorporated films (TIFNA) St. Louis, MO, USA, 1 wt% of 25 nm TiO2 nanoparticles (anatase phase, Sigma-Aldrich, St. Louis, MO, USA; 637254) was dispersed into the sol via probe ultrasonication for 1 h. The unincorporated TiO2 film (UTIF) was prepared without nanoparticles. Fluorine-doped tin oxide (FTO) glass substrates were cleaned in an ultrasonic bath with acetone before coating. The TiO2 sol was deposited by spin coating, followed by drying on a hotplate at 300 °C. This process was repeated five times to achieve uniform thickness, and the films were annealed in a furnace (Nabertherm L9/11, 600 °C, 2 h, 5 °C/min ramp rate) to enhance crystallinity.
The annealed TiO2 films (UTIF and TIFNA) were sensitized in 0.5 mM N719 dye (Ruthenizer 535-bisTBA, Solaronix SA, Aubonne, Switzerland) solution for 24 h at room temperature in the dark, rinsed with absolute ethanol, and assembled into DSSCs with a platinum-coated FTO counter electrode (prepared by thermal decomposition of 5 mM H2PtCl6 in isopropanol at 400 °C for 30 min) and an I−/I3− redox electrolyte (0.5 M LiI, 0.05 M I2, and 0.5 M 4-tert-butylpyridine in acetonitrile). The cells were sealed using a 60 μm Surlyn spacer (Solaronix, Meltonix 1170-60) with a hot press. Structural properties were analyzed using X-ray diffraction (XRD, Bruker D8 Advance, Bruker AXS GmbH, Karlsruhe, Germany; Cu-Kα radiation, λ = 1.5406 Å). Surface morphology and film thickness were examined by field-emission scanning electron microscopy (FE-SEM, JEOL JSM-7600F, 5 kV). Photovoltaic performance was evaluated under AM 1.5G simulated solar irradiation (Newport Oriel Sol3A Class AAA solar simulator, 100 mW/cm2), with current–voltage (I-V) characteristics recorded using a source meter (Keithley 2400, 4-wire mode). The active cell area was defined as 0.25 cm2 by a black metal mask.
3. Result and Discussion
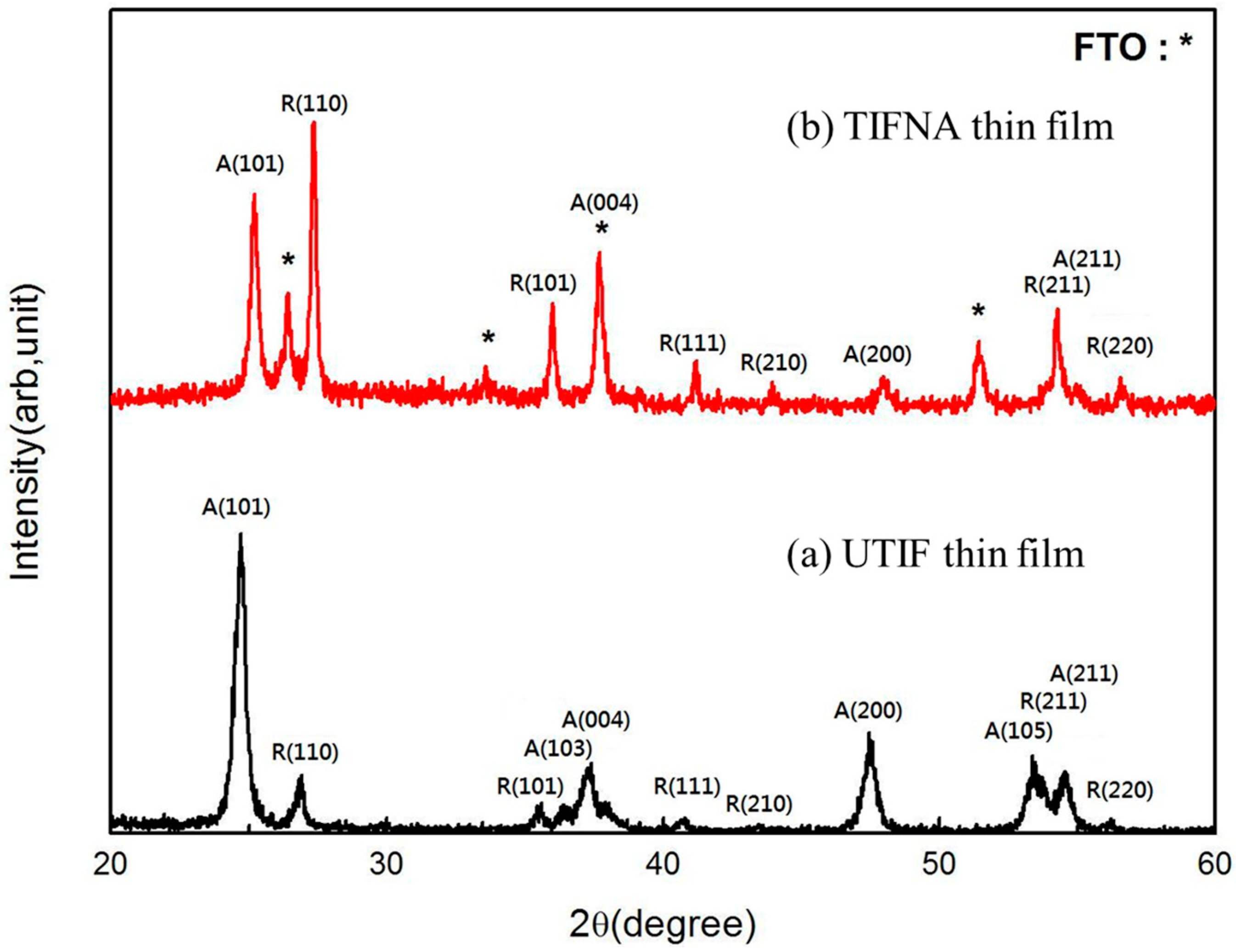
Figure 1 presents the X-ray diffraction (XRD) patterns of (a) UTIF and (b) TIFNA deposited on FTO substrates. The diffraction peaks are indexed to the anatase (A) and rutile (R) phases of TiO
2, with the FTO substrate peaks marked by asterisks (*). For the UTIF sample (
Figure 1a), prominent anatase peaks are observed at 2θ = 25.3° (A(101)), 37.8° (A(004)), and 48.0° (A(200)), confirming the formation of the anatase phase after annealing at 600 °C. Additionally, weaker rutile peaks appear at 27.4° (R(110)) and 36.1° (R(101)), indicating partial phase transformation. In contrast, the TIFNA sample (
Figure 1b) exhibits enhanced anatase crystallinity, as evidenced by the intensified A(101) peak. The incorporation of 25 nm TiO
2 nanoparticles appears to promote anatase stability, suppressing rutile formation. The rutile-related peaks (R(100), R(111), and R(220)) are less pronounced, suggesting that the nanoparticles act as nucleation sites for anatase growth. The absence of the A(110) peak in TIFNA, which is present in UTIF, further indicates modified crystallographic orientation due to nanoparticle inclusion. The crystallite sizes were estimated using the Scherrer equation applied to the A(101) peak, revealing a slight increase for TIFNA (~28 nm) compared to UTIF (~22 nm). This growth in crystallite size correlates with improved electron transport, as larger crystals reduce grain boundary scattering. The XRD results demonstrate that nanoparticle incorporation enhances anatase phase purity and crystallinity, which are critical for efficient dye adsorption and charge collection in DSSCs.
The surface morphologies of the TiO
2 thin films were examined using field-emission scanning electron microscopy (FE-SEM), as shown in
Figure 2. The unincorporated sol–gel derived film (UTIF,
Figure 2a) exhibits a smooth and compact surface composed of densely packed fine particles with relatively low surface roughness. The uniform morphology suggests limited surface area, which may restrict dye adsorption and hinder light harvesting efficiency in DSSCs. In contrast, the film incorporating 25 nm TiO
2 nanoparticles (TIFNA,
Figure 2b) presents a significantly rougher and more porous surface structure. The nanoparticles are discernible, forming a loosely aggregated network with interparticle spaces. This microstructure is advantageous for DSSC applications, as it provides a higher surface area for dye loading, promotes more effective light scattering, and facilitates faster electron transport pathways. These morphological improvements are expected to enhance the photovoltaic performance of TIFNA-based devices.
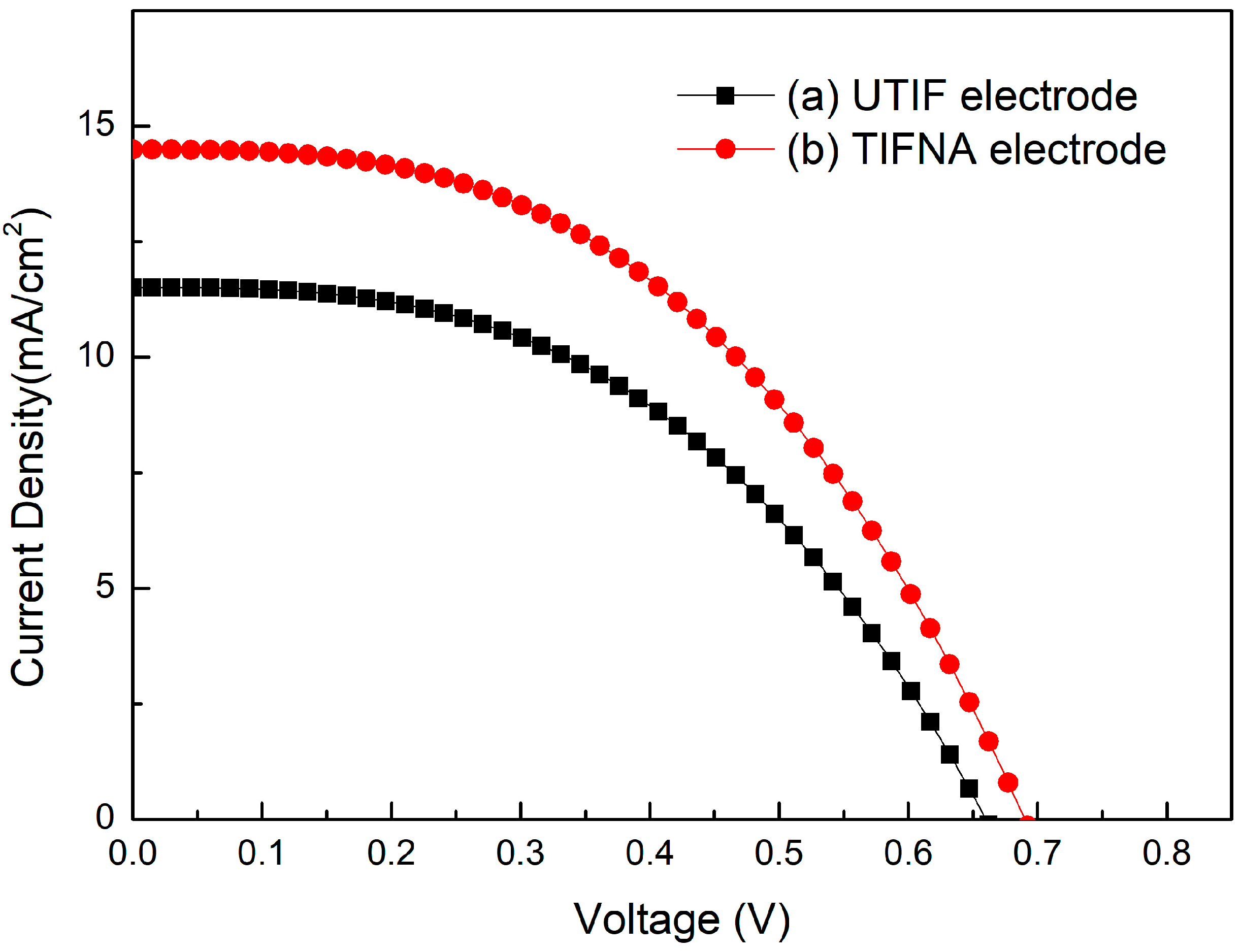
The I-V characteristics of DSSCs prepared using UTIF and TIFNA films are shown in
Figure 3, and the corresponding optoelectronic parameters are listed in
Table 1. Under AM 1.5G simulated sunlight (100 mW/cm
2), the DSSC with UTIF film has a
Jsc of 11.51 mA/cm
2, a
Voc of 0.66 V, a fill factor (
FF) of 55.7%, and an efficiency of 4.23%. The photovoltaic performance of DSSCs was significantly enhanced after the addition of TiO
2 nanoparticles (TIFNA). The TIFNA-based DSSC achieved a higher
Jsc of 14.49 mA/cm
2, an improved
Voc of 0.69 V, and a fill factor of 60.5%, resulting in a significant increase in the power conversion efficiency to 6.05%. The increase in
Jsc is mainly due to the improved dye adsorption and light scattering, while the enhancement in
Voc and
FF indicates better charge transport and suppressed recombination. These findings corroborate the morphological analysis, where TIFNA films exhibited a nanostructure more favorable for electron collection and dye absorption.
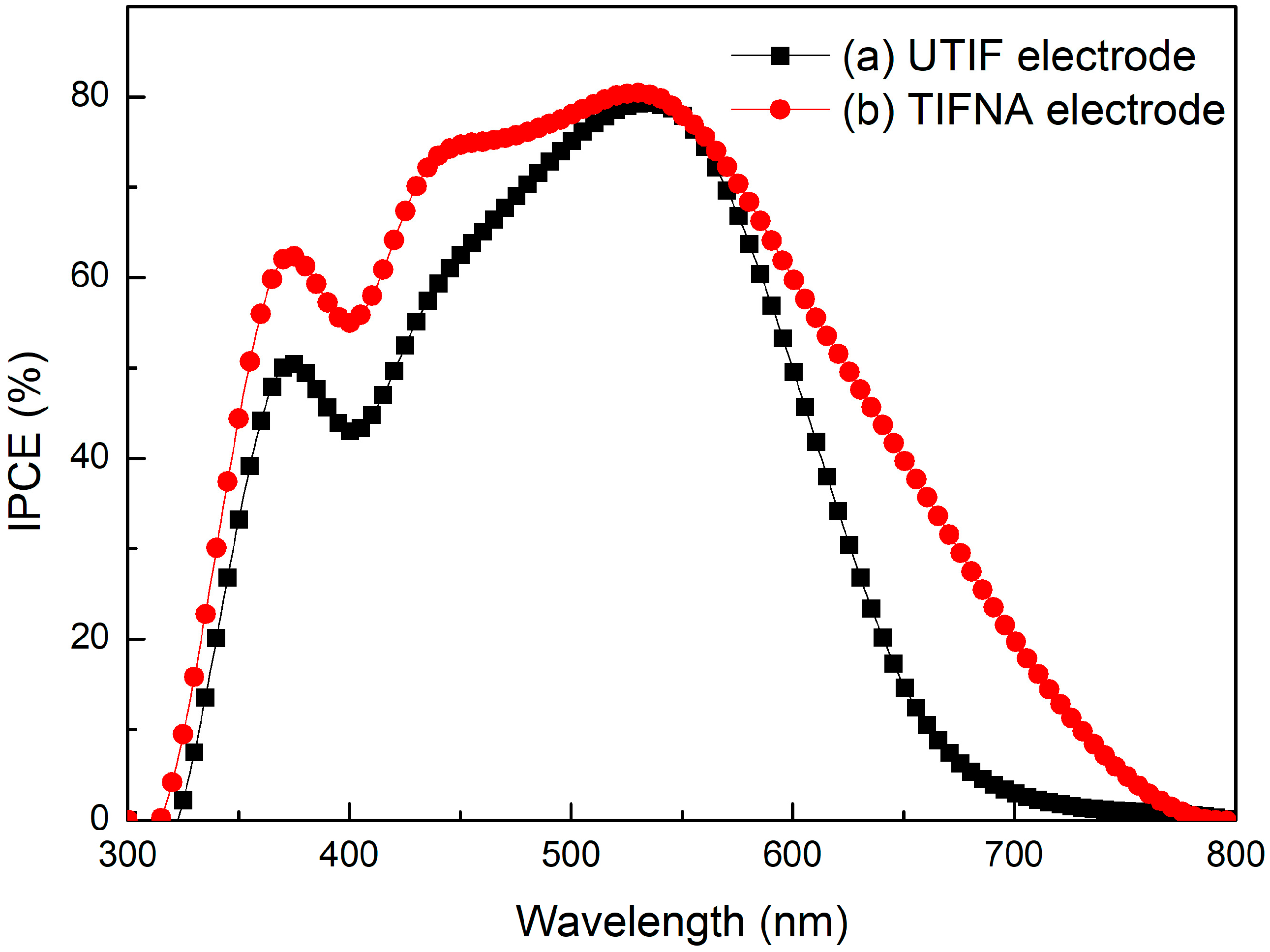
Figure 4 shows the incident photon-to-electron conversion efficiency (IPCE) spectra of DSSCs based on the UTIF and TIFNA electrodes. Compared with the UTIF-based device, the TIFNA-based DSSCs exhibited a significantly enhanced IPCE response over the visible spectrum (300–750 nm). The TIFNA electrode exhibited an IPCE maximum of about 82% around 520 nm, while the UTIF electrode reached a lower peak of about 78%. In addition, TIFNA maintained a high IPCE in both the short-wavelength range (300–400 nm) and the long-wavelength region above 600 nm. This more extensive and intense response indicates that the dye molecules adsorbed on the TIFNA film have more efficient photon absorption and charge injections. The improvement in IPCE was attributed to the addition of 25 nm TiO
2 nanoparticles, which improved the surface morphology and increased the dye loading. The rougher and more porous structure is conducive to better light scattering and dye penetration, thus achieving a more efficient photogenerated electron transfer process. These findings are consistent with the J-V data and support the observed enhancements in short-circuit current density (
Jsc) and overall photovoltaic efficiency.
4. Conclusions
In this study, TiO2 thin films with 25 nm TiO2 nanoparticles were successfully prepared by the sol–gel method. The experimental results showed that the TIFNA sample exhibited enhanced anatase crystallization and the addition of 25 nm TiO2 nanoparticles could promote the anatase phase and inhibit the formation of rutile. In addition, the addition of TiO2 nanoparticles significantly enhanced the surface roughness and porosity of the film, providing a larger effective surface area for dye adsorption. Photovoltaic measurements confirmed that the TIFNA device achieved a higher power conversion efficiency of 6.05% than 4.23% for the UTIF device. This improvement is mainly attributed to the increase in short-circuit current density (Jsc) and FF. The IPCE spectra showed that the TIFNA-based DSSCs exhibited higher conversion efficiency in both the short-wavelength (300–400 nm) and long-wavelength (> 600 nm) regions, with a maximum IPCE of approximately 82%.
Author Contributions
Conceptualization, M.-C.K. and K.-H.C.; methodology, M.-C.K. and C.-S.H.; formal analysis, M.-C.K., K.-H.C. and C.-S.H.; investigation, M.-C.K.; resources, K.-H.C.; data curation, M.-C.K.; writing—original draft preparation, K.-H.C. and C.-S.H.; writing—review and editing, M.-C.K.; visualization, M.-C.K. and K.-H.C.; project administration, M.-C.K. and K.-H.C.; funding acquisition, K.-H.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science Council of the Republic of China under grant number NSTC 114-2221-E-324-007.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to confidentiality agreement.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Abdullaev, S.S.; Breesam, Y.F.; AlZubaidi, A.A.H.; Tripathi, A.K.; Kareem, A.K.; Kuznetsov, S.V.; Alawsi, T.; Zabibah, R.S. ZnO@ZnCo2O4 core-shell: A novel high electrocatalytic nanostructure to replace platinum as the counter electrode in dye-sensitized solar cells. Mater. Sci. Semicond. Process. 2023, 165, 107709. [Google Scholar] [CrossRef]
- Wu, Y.; Lin, X.; Wang, H.; Lin, J. Counter electrodes in dye-sensitized solar cells. Chem. Soc. Rev. 2017, 46, 5975–6023. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Lin, Y.; Hagfeldt, A.; Grätzel, M. Mesoporous counter electrodes for dye-sensitized solar cells. Chem. Mater. 2010, 22, 579–584. [Google Scholar] [CrossRef]
- Kay, A.; Grätzel, M. Low cost photovoltaic modules based on dye sensitized nanocrystalline titanium dioxide and carbon powder. Sol. Energy Mater. Sol. Cells 1996, 44, 99–117. [Google Scholar] [CrossRef]
- Ito, A.; Murakami, S.; Kitamura, T.; Wada, Y.; Yanagida, S. Fabrication of a platinum nanocluster film by the Langmuir–Blodgett technique and its application to a counter electrode of a dye-sensitized solar cell. Chem. Commun. 2001, 4, 435–436. [Google Scholar]
- Park, J.H.; Ko, S.H.; Park, H.; Kim, D. Platinum-free counter electrodes based on carbon materials. Nanotechnology 2014, 25, 094007. [Google Scholar]
- Li, X.; Zhang, J.; Shen, Y.; Cai, M. Recent progress in metal sulfide counter electrodes for dye-sensitized solar cells. J. Power Sources 2015, 296, 2–22. [Google Scholar]
- Wang, H.; Wu, H.; Meng, L.; Ma, J. Nickel sulfide counter electrodes for dye-sensitized solar cells. J. Mater. Chem. A 2013, 1, 3407–3413. [Google Scholar]
- Huang, Y.J.; Lee, C.P.; Pang, H.W.; Li, C.T.; Fan, M.S. Microemulsion-controlled synthesis of CoSe2/CoSeO3 composite crystals for electrocatalysis in dye-sensitized solar cells. Mater. Today Energy 2017, 6, 189–197. [Google Scholar] [CrossRef]
- Du, F.; Yang, Q.; Qin, T.; Li, G. Morphology-controlled growth of NiCo2O4 ternary oxides and their application in dye-sensitized solar cells as counter electrodes. Sol. Energy 2017, 144, 7–13. [Google Scholar] [CrossRef]
- Jin, Z.; Zhao, G.; Wang, Z.S. Controllable growth of NixCoySe films and the influence of composition on the photovoltaic performance of quasi-solid-state dye-sensitized solar cells. J. Mater. Chem. C 2018, 6, 3901–3907. [Google Scholar] [CrossRef]
- Lu, M.; Lin, J.; Wei, T. Exploring the main function of reduced graphene oxide nano-flakes in a nickel cobalt sulfide counter electrode for dye-sensitized solar cell. J. Power Sources 2016, 336, 50–57. [Google Scholar] [CrossRef]
- Singh, E.; Kim, K.S.; Yeom, G.Y.; Nalwa, H.S. Two-dimensional transition metal dichalcogenides-based counter electrodes for dye-sensitized solar cells. Nanomaterials 2017, 7, 11. [Google Scholar] [CrossRef]
- Younas, M.; Baroud, T.N.; Gondal, M.A.; Dastageer, M.A.; Giannelis, E.P. Highly efficient, cost-effective counter electrodes for dye-sensitized solar cells (DSSCs) augmented by highly mesoporous carbons. J. Power Sources 2020, 448, 227375. [Google Scholar] [CrossRef]
- Zatirostami, A. Electro-deposited SnSe on ITO: A low-cost and high-performance counter electrode for DSSCs. J. Alloys Compd. 2020, 844, 156151. [Google Scholar] [CrossRef]
- Gullace, S.; Nastasi, F.; Puntoriero, F.; Trusso, S.; Calogero, G. A platinum-free nanostructured gold counter electrode for DSSCs prepared by pulsed laser ablation. Appl. Surf. Sci. 2020, 527, 146797. [Google Scholar] [CrossRef]
- Mehmood, U.; Khan, A.U.H. Spray coated PbS nano-crystals as an effective counter-electrode material for platinum free dye-sensitized solar cells (DSSCs). Sol. Energy 2019, 188, 1113–1121. [Google Scholar] [CrossRef]
- Ayaz, M.; Hijji, M.; Alatawi, A.S.; Namazi, M.A.; Ershath, M.I.M. Enhancing photovoltaic performance in dye-sensitized solar cells using nanostructured NiS/MoS2 composite counter electrodes. Mater. Sci. Semicond. Process. 2024, 165, 108172. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).