Multiplexed Quantification of Soil Nutrients Using an AI-Enhanced and Low-Cost Impedimetric Sensor †
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plant Growth Conditions
2.3. Device Design
2.4. Implantation Procedure and Probe Design
2.5. Data Acquisition and AI Modelling
3. Discussions
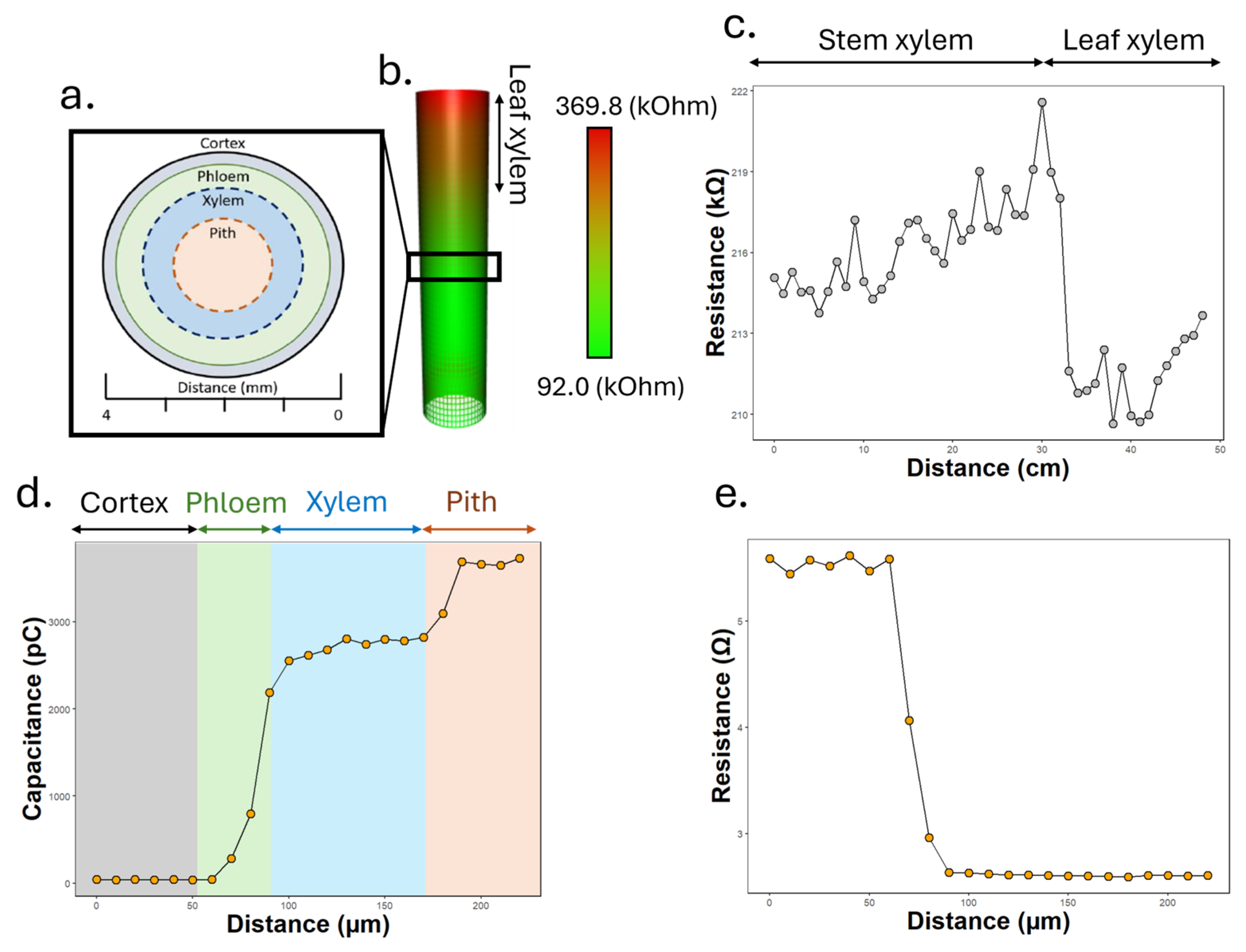
3.1. Impedance-Guided Localisation of Xylem Sap
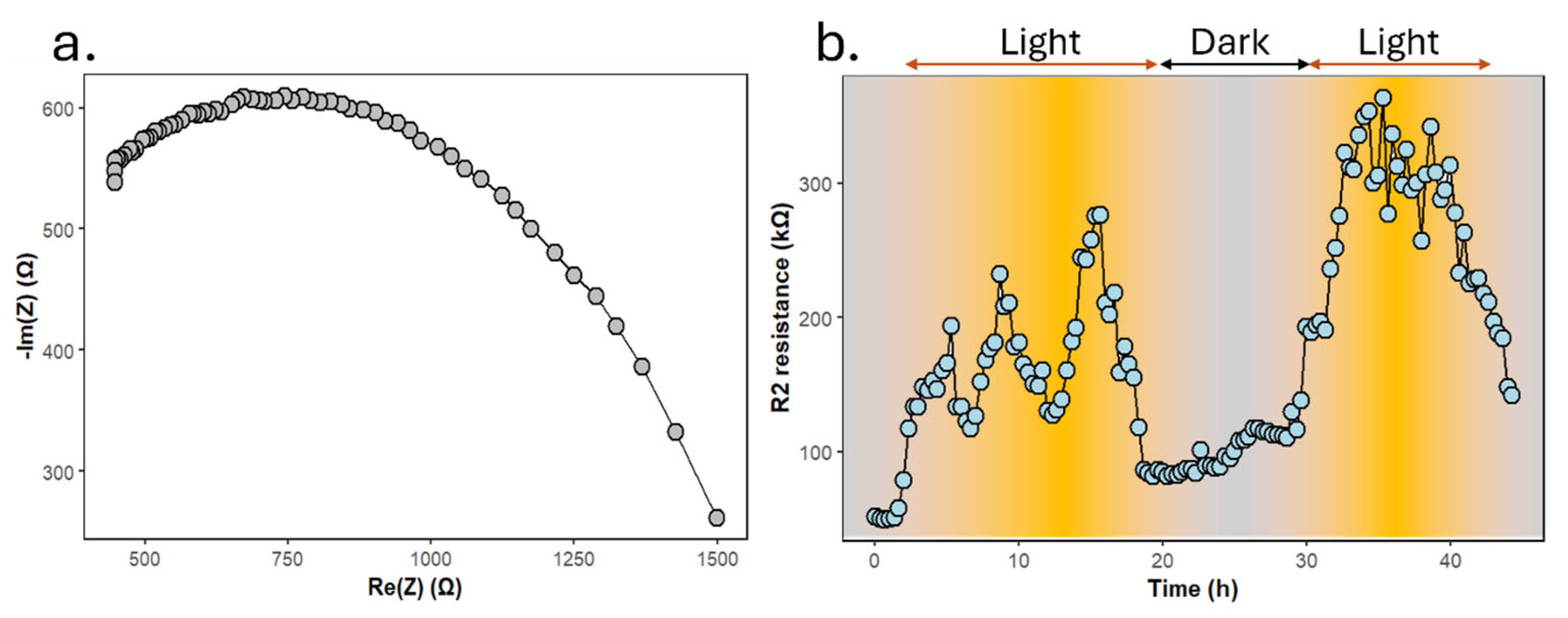
3.2. Electrolyte Monitoring via Impedance Spectroscopy
4. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Conflicts of Interest
References
- FAO. How to Feed the World in 2050. In Proceedings of the Expert Meeting on How to Feed the World in 2050, Rome, Italy, 24–26 June 2009. [Google Scholar]
- van Dijk, M.; Morley, T.; Rau, M.L.; Saghai, Y. A meta-analysis of projected global food demand and population at risk of hunger for the period 2010–2050. Nat. Food 2021, 2, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.K.; Purohit, S.; Alam, E.; Islam, M.K. Advancements in soil management: Optimizing crop production through interdisciplinary approaches. J. Agric. Food Res. 2024, 18, 101528. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Nahar, K.; Hossain, M.S.; Mahmud, J.A.; Hossen, M.S.; Masud, A.A.C.; Moumita; Fujita, M. Potassium: A Vital Regulator of Plant Responses and Tolerance to Abiotic Stresses. Agronomy 2018, 8, 31. [Google Scholar] [CrossRef]
- Zhou, H.; Shi, H.; Yang, Y.; Feng, X.; Chen, X.; Xiao, F.; Lin, H.; Guo, Y. Insights into plant salt stress signaling and tolerance. J. Genet. Genom. 2024, 51, 16–34. [Google Scholar] [CrossRef]
- Gupta, S.; Kaur, N.; Kant, K.; Jindal, P.; Ali, A.; Naeem, M. Calcium: A master regulator of stress tolerance in plants. S. Afr. J. Bot. 2023, 163, 580–594. [Google Scholar] [CrossRef]
- Ragel, P.; Raddatz, N.; Leidi, E.O.; Quintero, F.J.; Pardo, J.M. Regulation of K+ Nutrition in Plants. Front. Plant Sci. 2019, 10, 281. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.; Chen, X.; Liu, S.; Zhang, W. Effects of Temperature and Moisture on Soil Organic Carbon Mineralization. IOP Conf. Ser. Mater. Sci. Eng. 2019, 562, 012085. [Google Scholar]
- Zhai, J.; Luo, B.; Li, A.; Dong, H.; Jin, X.; Wang, X. Unlocking All-Solid Ion Selective Electrodes: Prospects in Crop Detection. Sensors 2022, 22, 5541. [Google Scholar] [CrossRef]
- Saha, A.; Mi, Y.; Glassmaker, N.; Shakouri, A.; Alam, M.A. In Situ Drift Monitoring and Calibration of Field-Deployed Potentiometric Sensors Using Temperature Supervision. ACS Sens. 2023, 8, 2799–2808. [Google Scholar] [CrossRef]
- Mustafa, R.; Ansari, A. Comprehensive analysis of soil electrical conductivity: An experimental and machine learning approach. Discov. Civ. Eng. 2024, 1, 82. [Google Scholar] [CrossRef]
- Su, S.L.; Singh, D.N.; Baghini, M.S. A critical review of soil moisture measurement. Measurement 2014, 54, 92–105. [Google Scholar] [CrossRef]
- Qin, J.; Monje, O.; Nugent, M.R.; Finn, J.R.; O’Rourke, A.E.; Wilson, K.D.; Fritsche, R.F.; Baek, I.; Chan, D.E.; Kim, M.S. A hyperspectral plant health monitoring system for space crop production. Front. Plant Sci. 2023, 14, 1133505. [Google Scholar] [CrossRef] [PubMed]
- Rengasamy, P. Soil Chemistry Factors Confounding Crop Salinity Tolerance—A Review. Agronomy 2016, 6, 53. [Google Scholar] [CrossRef]
- Adamchuk, V.I.; Hummel, J.W.; Morgan, M.T.; Upadhyaya, S.K. On-the-go soil sensors for precision agriculture. Comput. Electron. Agric. 2004, 44, 71–91. [Google Scholar] [CrossRef]
- Rossel, R.A.V.; Chen, C. Digitally mapping the information content of visible–near infrared spectra of surficial Australian soils. Remote Sens. Environ. 2011, 115, 1443–1455. [Google Scholar] [CrossRef]
- Islam, M.R.; Oliullah, K.; Kabir, M.M.; Alom, M.; Mridha, M.F. Machine learning enabled IoT system for soil nutrients monitoring and crop recommendation. J. Agric. Food Res. 2023, 14, 100880. [Google Scholar] [CrossRef]
- Cho, W.-J.; Kim, H.-J.; Jung, D.-H.; Han, H.-J.; Cho, Y.-Y. Hybrid Signal-Processing Method Based on Neural Network for Prediction of NO3, K, Ca, and Mg Ions in Hydroponic Solutions Using an Array of Ion-Selective Electrodes. Sensors 2019, 19, 5508. [Google Scholar] [CrossRef] [PubMed]
- Babu, G.R.; Gokuldhev, M.; Brahmanandam, P.S. Integrating IoT for Soil Monitoring and Hybrid Machine Learning in Predicting Tomato Crop Disease in a Typical South India Station. Sensors 2024, 24, 6177. [Google Scholar] [CrossRef]
- Freeborn, T.J.; Elwakil, A.; Maundy, B. Electrode location impact on cole-impedance parameters using magnitude-only measurements. In Proceedings of the 2016 IEEE 59th International Midwest Symposium on Circuits and Systems (MWSCAS), Abu Dhabi, United Arab Emirates, 16–19 October 2016. [Google Scholar]
- Muñoz, J.D.; Mosquera, V.H.; Rengifo, C.F. A low-cost, portable, two-dimensional bioimpedance distribution estimation system based on the AD5933 impedance converter. HardwareX 2022, 11, e00274. [Google Scholar] [CrossRef]
- Freeborn, T.J.; Elwakil, A.S.; Maundy, B. Variability of Cole-model bioimpedance parameters using magnitude-only measurements of apples from a two-electrode configuration. Int. J. Food Prop. 2017, 20 (Suppl. S1), S507–S519. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, Q.; Liu, M.; Zhou, H.; Ma, C.; Wang, P. Regulation of Plant Responses to Salt Stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Du, X.; Wang, F.; Sha, J.; Chen, Q.; Tian, G.; Zhu, Z.; Ge, S.; Jiang, Y. Effects of Potassium Levels on Plant Growth, Accumulation and Distribution of Carbon, and Nitrate Metabolism in Apple Dwarf Rootstock Seedlings. Front. Plant Sci. 2020, 11, 904. [Google Scholar] [CrossRef] [PubMed]
- Benson, K.; Cramer, S.; Galla, H.-J. Impedance-based cell monitoring: Barrier properties and beyond. Fluids Barriers CNS 2013, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Abasi, S.; Aggas, J.R.; Garayar-Leyva, G.G.; Walther, B.K.; Guiseppi-Elie, A. Bioelectrical Impedance Spectroscopy for Monitoring Mammalian Cells and Tissues under Different Frequency Domains: A Review. ACS Meas. Sci. Au 2022, 2, 495–516. [Google Scholar] [CrossRef]
- Hussain, M.I.; El-Keblawy, A.; Akhtar, N.; Elwakil, A.S. Electrical Impedance Spectroscopy in Plant Biology. In Sustainable Agriculture Reviews 52; Lichtfouse, E., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 395–416. [Google Scholar]
- Liu, Y.; Li, D.; Qian, J.; Di, B.; Zhang, G.; Ren, Z. Electrical impedance spectroscopy (EIS) in plant roots research: A review. Plant Methods 2021, 17, 118. [Google Scholar] [CrossRef]
- Yang, L.; Liu, H.; Cohen, S.; Gao, Z. Microclimate and Plant Transpiration of Tomato (Solanum lycopersicum L.) in a Sunken Solar Greenhouse in North China. Agriculture 2022, 12, 260. [Google Scholar] [CrossRef]
- Lanoue, J.; Leonardos, E.D.; Grodzinski, B. Effects of Light Quality and Intensity on Diurnal Patterns and Rates of Photo-Assimilate Translocation and Transpiration in Tomato Leaves. Front. Plant Sci. 2018, 9, 756. [Google Scholar] [CrossRef]
- Fernández, J.E. Plant-Based Methods for Irrigation Scheduling of Woody Crops. Horticulturae 2017, 3, 35. [Google Scholar] [CrossRef]
- Azzarello, E.; Mugnai, S.; Pandolfi, C.; Masi, E.; Mancuso, S. Stress Assessment in Plants by Impedance Spectroscopy. Floric. Ornam. Plant Biotechnol. 2006, 3, 140–148. [Google Scholar]
- Huang, B.F.F.; Boutros, P.C. The parameter sensitivity of random forests. BMC Bioinform. 2016, 17, 331. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, W.-H. Potassium Transport and Signaling in Higher Plants. Annu. Rev. Plant Biol. 2013, 64, 451–476. [Google Scholar] [CrossRef] [PubMed]
- Van Haeverbeke, M.; De Baets, B.; Stock, M. Plant impedance spectroscopy: A review of modeling approaches and applications. Front. Plant Sci. 2023, 14, 1187573. [Google Scholar] [CrossRef] [PubMed]
- Ozier-Lafontaine, H.; Bajazet, T. Analysis of Root Growth by Impedance Spectroscopy (EIS). Plant Soil 2005, 277, 299–313. [Google Scholar] [CrossRef]

| Electrolyte | Model | R2 | MAE | RMSE |
|---|---|---|---|---|
| Sodium | Random Forest | 0.983 ± 0.005 | 0.939 ± 0.117 | 5.378 ± 0.716 |
| Potassium | 0.994 ± 0.004 | 0.345 ± 0.083 | 2.787 ± 0.830 | |
| Sodium | SVM | 0.132 ± 0.055 | 20.609 ± 1.122 | 38.795 ± 2.359 |
| Potassium | 0.493 ± 0.040 | 11.589 ± 0.622 | 25.960 ± 0.963 | |
| Sodium | XBoost | 0.989 ± 0.004 | 0.944 ± 0.093 | 4.336 ± 0.733 |
| Potassium | 0.995 ± 0.002 | 0.415 ± 0.081 | 2.554 ± 0.638 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Gonzalez, A. Multiplexed Quantification of Soil Nutrients Using an AI-Enhanced and Low-Cost Impedimetric Sensor. Eng. Proc. 2025, 106, 7. https://doi.org/10.3390/engproc2025106007
Ruiz-Gonzalez A. Multiplexed Quantification of Soil Nutrients Using an AI-Enhanced and Low-Cost Impedimetric Sensor. Engineering Proceedings. 2025; 106(1):7. https://doi.org/10.3390/engproc2025106007
Chicago/Turabian StyleRuiz-Gonzalez, Antonio. 2025. "Multiplexed Quantification of Soil Nutrients Using an AI-Enhanced and Low-Cost Impedimetric Sensor" Engineering Proceedings 106, no. 1: 7. https://doi.org/10.3390/engproc2025106007
APA StyleRuiz-Gonzalez, A. (2025). Multiplexed Quantification of Soil Nutrients Using an AI-Enhanced and Low-Cost Impedimetric Sensor. Engineering Proceedings, 106(1), 7. https://doi.org/10.3390/engproc2025106007





