Microwave-Assisted Synthesis of Aryl Phosphonates and Tertiary Phosphine Oxides by the Hirao Reaction †
Abstract
:1. Introduction
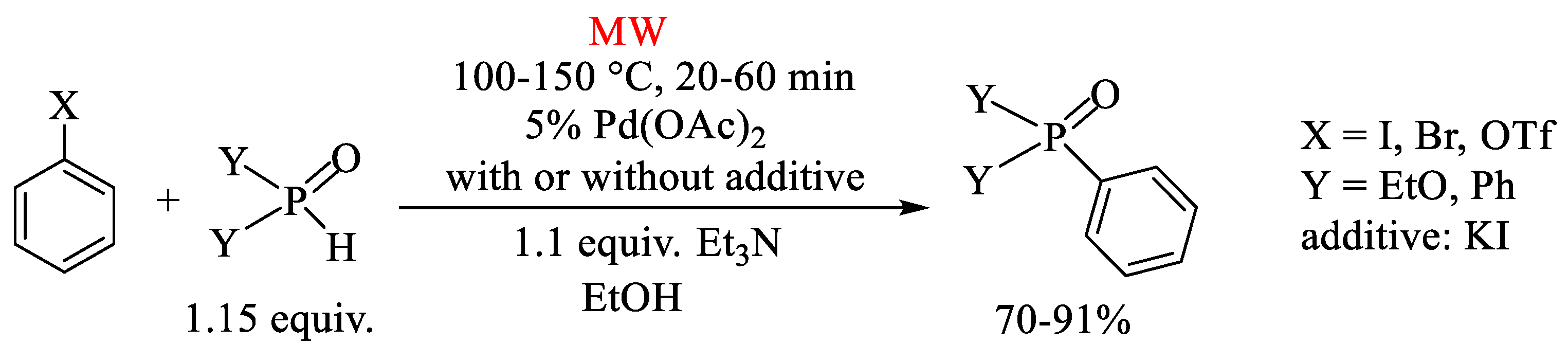
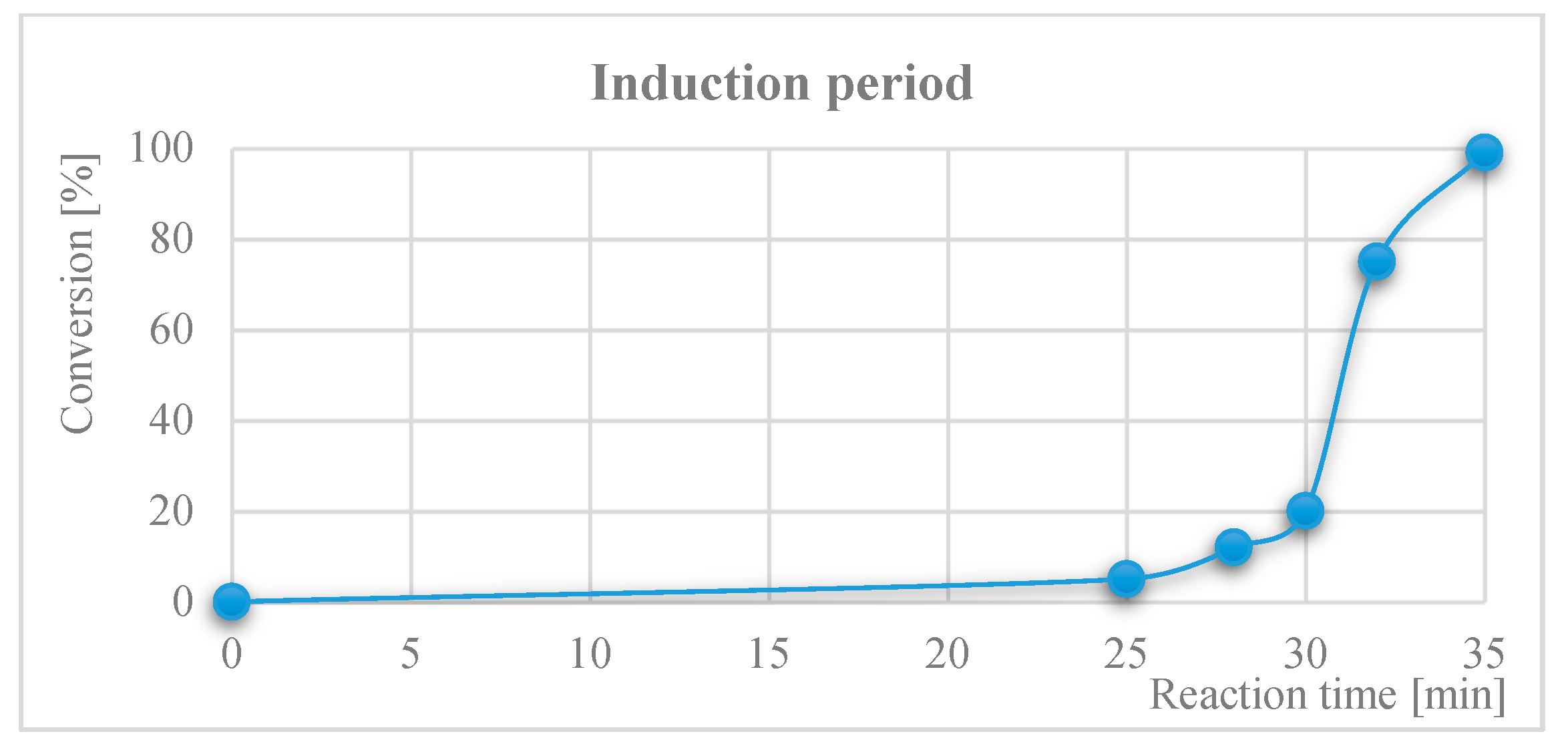
2. Pd(OAc)2-Catalyzed P–C Coupling Reactions in the Absence of Directly Added P-Ligands
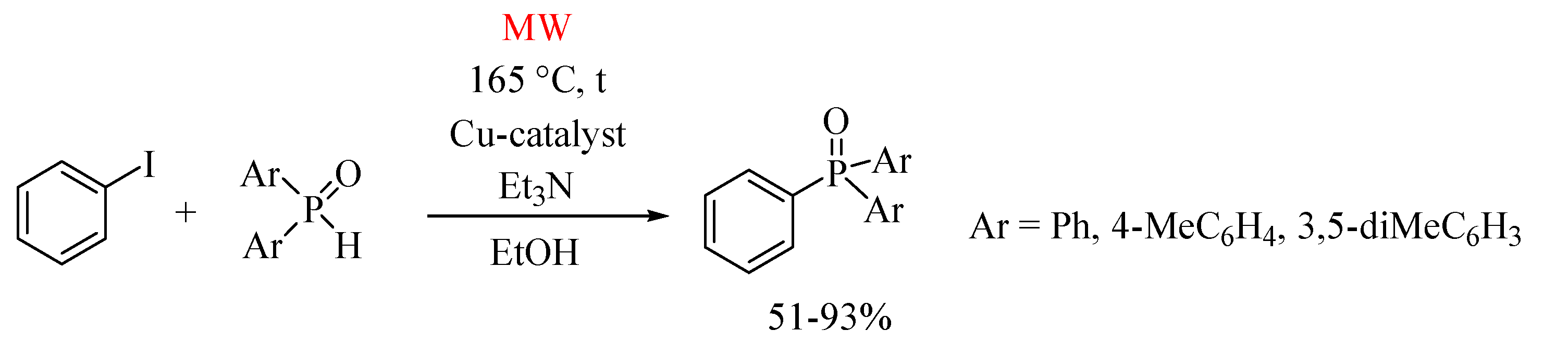
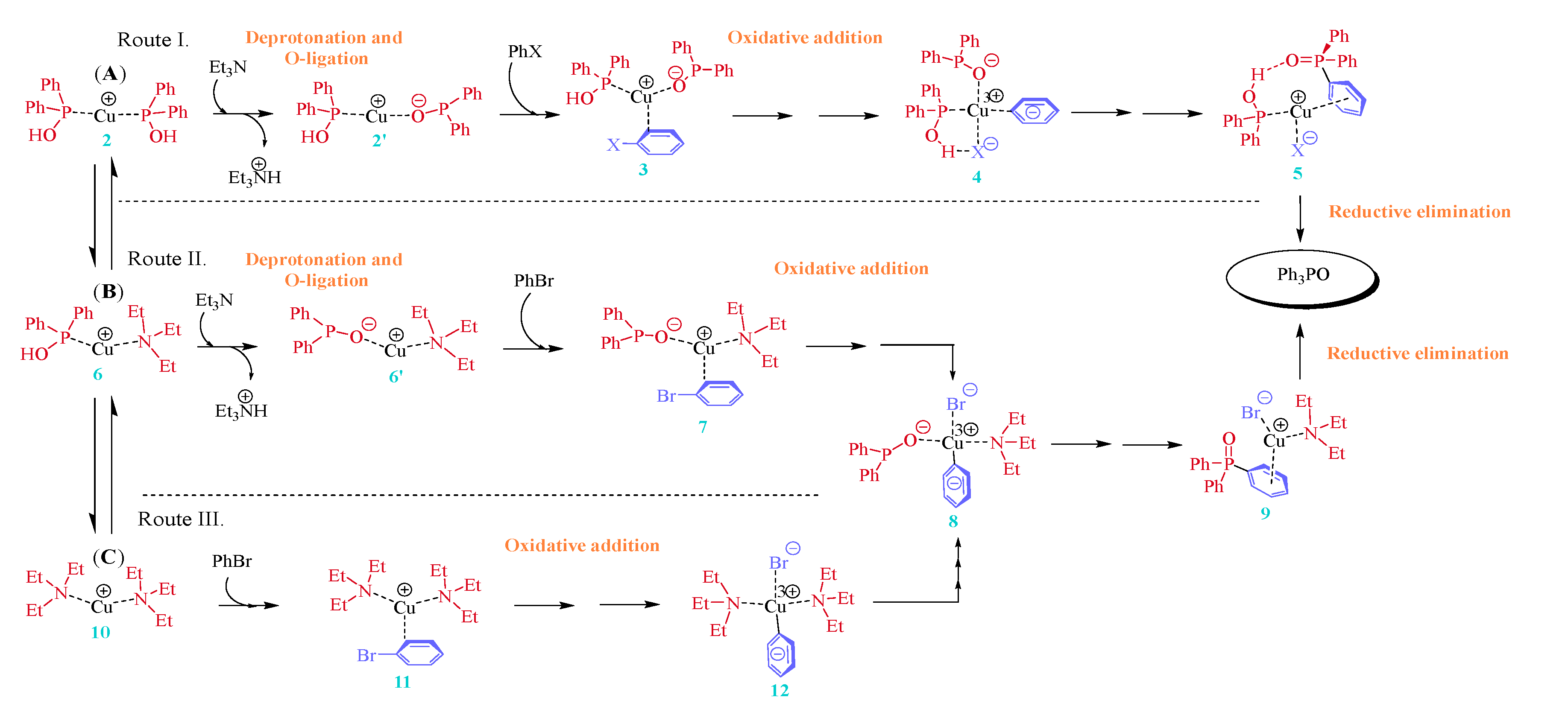
3. Cu(I)-Catalyzed Hirao Reaction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Keglevich, G. Organophosphorus Chemistry—Novel Developments; De Gruyter: Berlin, Germany, 2018. [Google Scholar] [CrossRef] [Green Version]
- Hirao, T.; Masunaga, T.; Ohshiro, Y.; Agawa, T. Stereoselective synthesis of vinylphosphonate. Tetrahedron Lett. 1980, 21, 3595–3598. [Google Scholar] [CrossRef]
- Hirao, T.; Masunaga, T.; Yamada, N.; Ohshiro, Y.; Agawa, T. Palladium-catalyzed new carbon-phosphorus bond formation. Bull. Chem. Soc. Jpn. 1982, 55, 909–913. [Google Scholar] [CrossRef] [Green Version]
- Jablonkai, E.; Keglevich, G. Advances and new variations of the Hirao reaction. Org. Prep. Proced. Int. 2014, 46, 281–316. [Google Scholar] [CrossRef]
- Jablonkai, E.; Keglevich, G. P–C bond formation by coupling reaction utilizing >P(O)H species as the reagents. Curr. Org. Synth. 2014, 11, 429–453. [Google Scholar] [CrossRef]
- Henyecz, R.; Keglevich, G. New developments on the Hirao reactions, especially from ”green” point of view. Curr. Org. Synth. 2019, 16, 523–545. [Google Scholar] [CrossRef]
- Jablonkai, E.; Keglevich, G. P-ligand-free, microwave-assisted variation of the Hirao reaction under solvent-free conditions; the P–C coupling reaction of >P(O)H species and bromoarenes. Tetrahedron Lett. 2013, 54, 4185–4188. [Google Scholar] [CrossRef]
- Keglevich, G.; Jablonkai, E.; Balázs, L.B. A “green” variation of the Hirao reaction: The P–C coupling of diethyl phosphite, alkyl phenyl-H-phosphinates and secondary phosphine oxides with bromoarenes using P-ligand-free Pd(OAc)2 catalyst under microwave and solvent-free conditions. RSC Adv. 2014, 4, 22808–22816. [Google Scholar] [CrossRef] [Green Version]
- Kalek, M.; Stawinski, J. Pd(0)-catalyzed phosphorus-carbon bond formation. Mechanistic and synthetic studies on the role of the palladium sources and anionic additives. Organometallics 2007, 26, 5840–5847. [Google Scholar] [CrossRef]
- Kalek, M.; Stawinski, J. Palladium-catalyzed C–P bond formation: Mechanistic studies on the ligand substitutio and the reductive elimination. An intramolecular catalysis by the acetate group in PdII complexes. Organometallics 2008, 27, 5876–5888. [Google Scholar] [CrossRef]
- Keglevich, G.; Henyecz, R.; Mucsi, Z.; Kiss, N.Z. The palladium acetate-catalyzed microwave-assisted Hirao reaction without an added phosphorus ligand as a “green” protocol: A quantum chemical study on the mechanism. Adv. Synth. Catal. 2017, 359, 4322–4331. [Google Scholar] [CrossRef]
- Henyecz, R.; Mucsi, Z.; Keglevich, G. Palladium-catalyzed microwave-assisted Hirao reaction utilizing the excess of the diarylphosphine oxide reagent as the P-ligand; a study on the activity and formation of the “PdP2” catalyst. Pure Appl. Chem. 2019, 91, 121–134. [Google Scholar] [CrossRef]
- Henyecz, R.; Huszár, B.; .Grenitzer, V.; Keglevich, G. A study on the reactivity of monosubstituted bentenes in the MW-assisted Pd(OAc)2-catalyzed Hirao reaction with Ph2P(O) and (EtO)2P(O)H reagents. Curr. Org. Chem. 2020, 24, 1048–1054. [Google Scholar] [CrossRef]
- Jablonkai, E.; Balázs, L.B.; Keglevich, G. A P-ligand-free nickel-catalyzed variation of the Hirao reaction under microwave conditions. Curr. Org. Chem. 2015, 19, 197–202. [Google Scholar] [CrossRef]
- Henyecz, R.; Mucsi, Z.; Keglevich, G. A surprising mechanism lacking the Ni(0) state during the Ni(II)-catalyzed P–C cross-coupling reaction performed in the absence of a reducing agent—An experimental and a theoretical study. Pure Appl. Chem. 2020, 92, 493–503. [Google Scholar] [CrossRef] [Green Version]
- Keglevich, G.; Henyecz, R.; Mucsi, Z. Experimental and theoretical study on the “2,2′-bipiridyl-Ni-catalyzed” Hirao reaction of >P(O)H reagents and halobenzenes: A Ni(0)→Ni(II) or a Ni(II)→Ni(IV) mechanism? J. Org. Chem. 2020, 85, 14486–14495. [Google Scholar] [CrossRef]
- Keglevich, G.; Henyecz, R.; Mucsi, Z. Focusing on the catalysts of the Pd- and Ni-catalyzed Hirao reactions. Molecules 2020, 25, 3897. [Google Scholar] [CrossRef]
- Huszár, B.; Henyecz, R.; Mucsi, Z.; Keglevich, G. MW-promoted Cu(I)-catalyzed P–C coupling reactions without the addition of conventional ligands; an experimental and a theoretical study. Catalysts 2021, 11, 933. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huszár, B.; Mucsi, Z.; Keglevich, G. Microwave-Assisted Synthesis of Aryl Phosphonates and Tertiary Phosphine Oxides by the Hirao Reaction. Chem. Proc. 2022, 8, 79. https://doi.org/10.3390/ecsoc-25-11697
Huszár B, Mucsi Z, Keglevich G. Microwave-Assisted Synthesis of Aryl Phosphonates and Tertiary Phosphine Oxides by the Hirao Reaction. Chemistry Proceedings. 2022; 8(1):79. https://doi.org/10.3390/ecsoc-25-11697
Chicago/Turabian StyleHuszár, Bianka, Zoltán Mucsi, and György Keglevich. 2022. "Microwave-Assisted Synthesis of Aryl Phosphonates and Tertiary Phosphine Oxides by the Hirao Reaction" Chemistry Proceedings 8, no. 1: 79. https://doi.org/10.3390/ecsoc-25-11697
APA StyleHuszár, B., Mucsi, Z., & Keglevich, G. (2022). Microwave-Assisted Synthesis of Aryl Phosphonates and Tertiary Phosphine Oxides by the Hirao Reaction. Chemistry Proceedings, 8(1), 79. https://doi.org/10.3390/ecsoc-25-11697






