Tropomyosin Analysis in Foods Using an Electrochemical Immunosensing Approach †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instrumentation
2.2. Reagents and Solutions
2.3. Sample Preparation
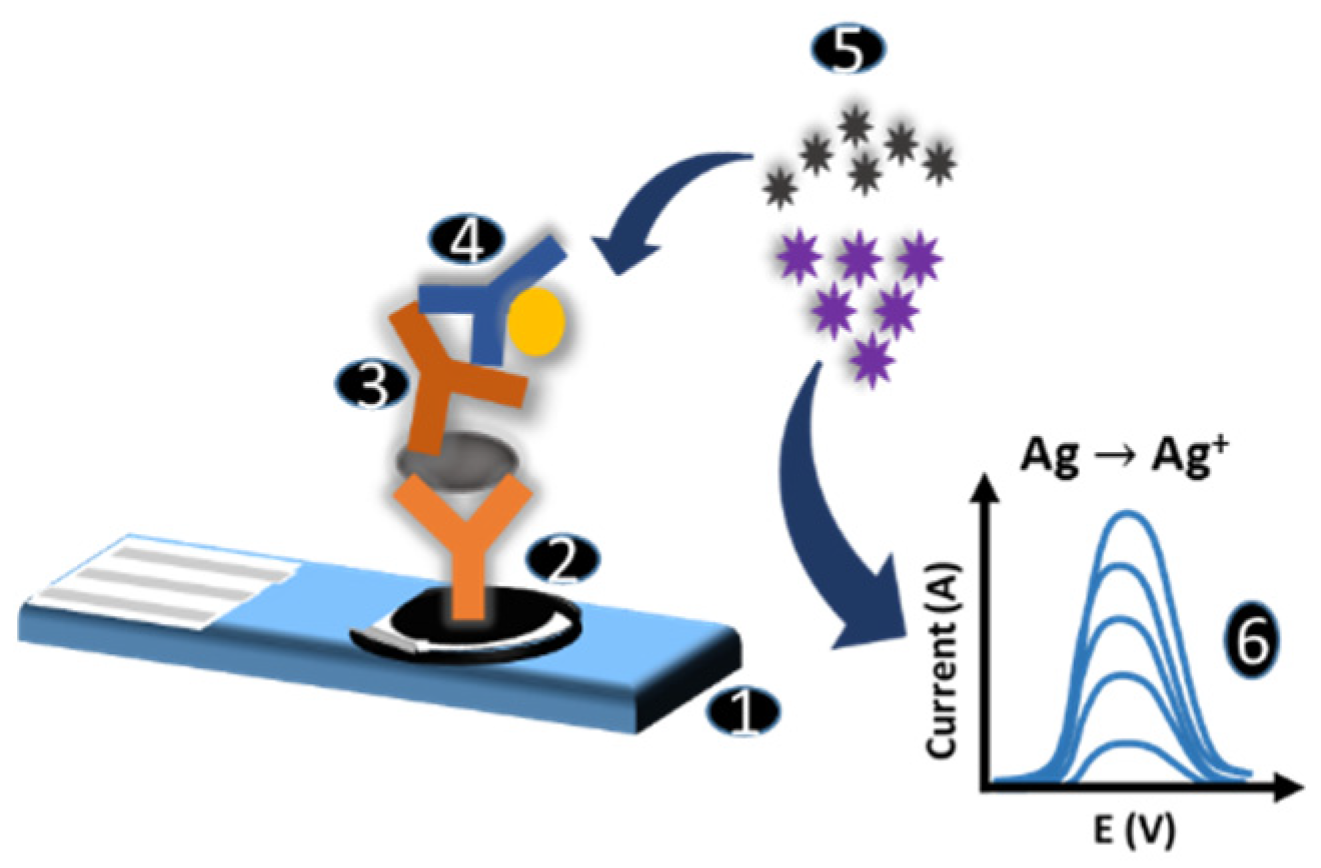
2.4. Immunosensor Assay and Electrochemical Measurements
3. Results and Discussion
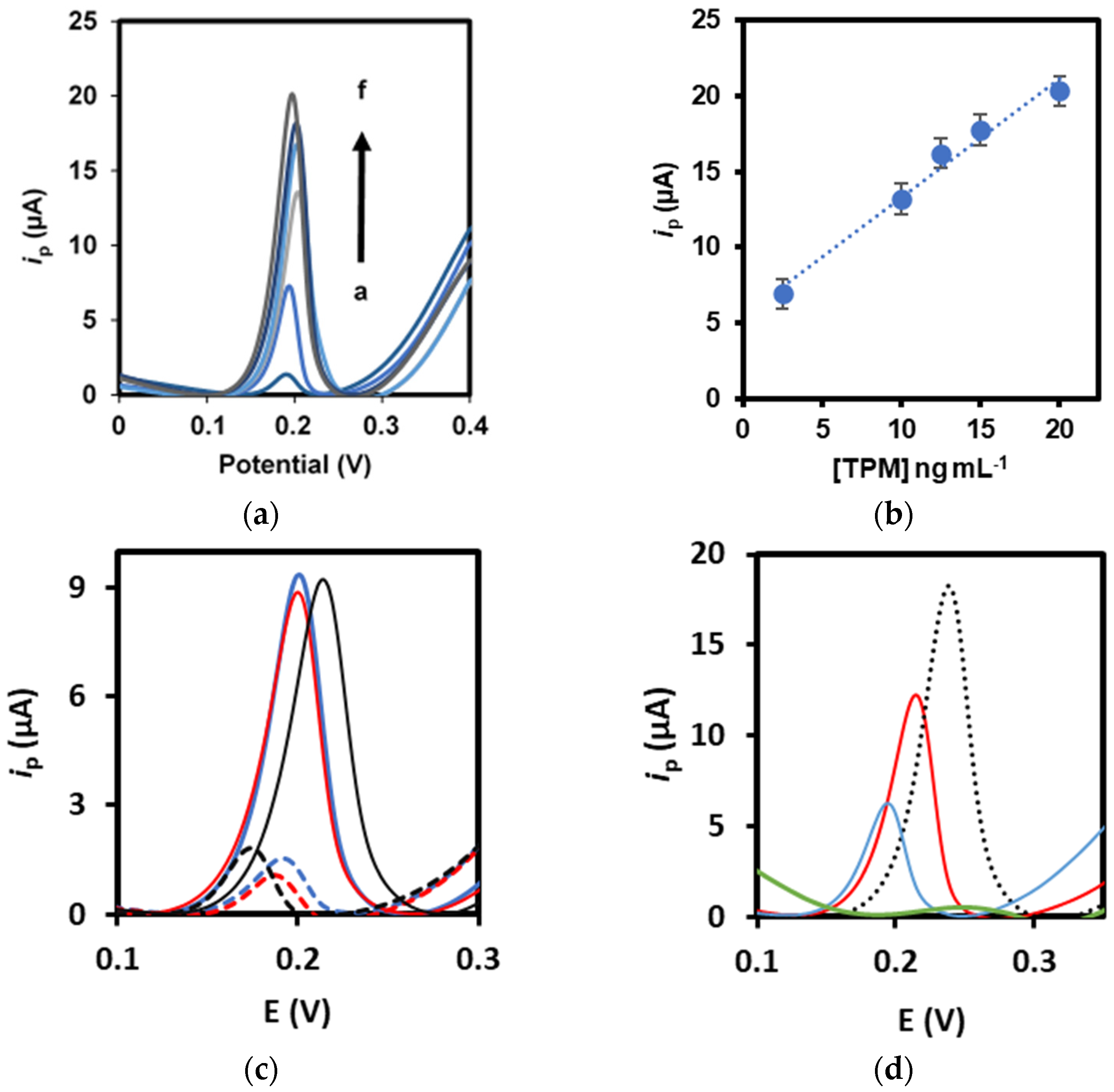
3.1. Optimization Studies
3.2. Analytical Performance
3.3. Selectivity and Interference Studies
3.4. Applicability to Food Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, D.; Ji, J.; Sun, X.; Zhang, Y.; Zhang, G.; Tang, L. Mast cell-based electrochemical biosensor for quantification of the major shrimp allergen Pen a 1 (tropomyosin). Biosens. Bioelectron. 2013, 50, 150–156. [Google Scholar] [CrossRef]
- Angulo-Ibáñez, A.; Eletxigerra, U.; Lasheras, X.; Campuzano, S.; Merino, S. Electrochemical tropomyosin allergen immunosensor for complex food matrix analysis. Anal. Chim. Acta 2019, 1079, 94–102. [Google Scholar] [CrossRef]
- Wang, Y.; Rao, Z.; Zhou, J.; Zheng, L.; Fu, L. A chiral assembly of gold nanoparticle trimer-based biosensors for ultrasensitive detection of the major allergen tropomyosin in shellfish. Biosens. Bioelectron. 2019, 132, 84–89. [Google Scholar] [CrossRef]
- Mohamad, A.; Rizwan, M.; Keasberry, N.A.; Nguyen, A.S.; Lam, T.D.; Ahmed, M.U. Gold-microrods/Pd-nanoparticles/polyaniline-nanocomposite-interface as a peroxidase-mimic for sensitive detection of tropomyosin. Biosens. Bioelectron. 2020, 155, 112108. [Google Scholar] [CrossRef]
- Chinnappan, R.; Rahamn, A.A.; AlZabn, R.; Kamath, S.; Lopata, A.L.; Abu-Salah, K.M.; Zourob, M. Aptameric biosensor for the sensitive detection of major shrimp allergen, tropomyosin. Food Chem. 2020, 314, 126133. [Google Scholar] [CrossRef] [PubMed]
- Fanjul-Bolado, P.; Hernández-Santos, D.; González-García, M.B.; Costa-García, A. Alkaline Phosphatase-Catalyzed Silver Deposition for Electrochemical Detection. Anal. Chem. 2007, 79, 5272–5277. [Google Scholar] [CrossRef] [PubMed]
- Neves, M.M.P.S.; González-García, M.B.; Nouws, H.P.A.; Costa-García, A. An electrochemical deamidated gliadin antibody immunosensor for celiac disease clinical diagnosis. Analyst 2013, 138, 1956–1958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neves, M.M.P.S.; González-García, M.B.; Nouws, H.P.A.; Costa-García, A. Celiac disease detection using a transglutaminase electrochemical immunosensor fabricated on nanohybrid screen-printed carbon electrodes. Biosens. Bioelectron. 2012, 31, 95–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Variable | Studied Range | Selected Value |
|---|---|---|
| (C-Ab), µg mL−1 | 2.5–20 | 20 |
| (D-Ab), dilution | 1:250–1:12,000 | 1:2000 |
| (AP-Ab), dilution | 1:10,000–1:40,000 | 1:40,000 |
| AP-Ab incubation time, min | 15–60 | 60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torre, R.; Freitas, M.; Costa-Rama, E.; Nouws, H.P.A.; Delerue-Matos, C. Tropomyosin Analysis in Foods Using an Electrochemical Immunosensing Approach. Chem. Proc. 2021, 5, 62. https://doi.org/10.3390/CSAC2021-10471
Torre R, Freitas M, Costa-Rama E, Nouws HPA, Delerue-Matos C. Tropomyosin Analysis in Foods Using an Electrochemical Immunosensing Approach. Chemistry Proceedings. 2021; 5(1):62. https://doi.org/10.3390/CSAC2021-10471
Chicago/Turabian StyleTorre, Ricarda, Maria Freitas, Estefanía Costa-Rama, Henri P. A. Nouws, and Cristina Delerue-Matos. 2021. "Tropomyosin Analysis in Foods Using an Electrochemical Immunosensing Approach" Chemistry Proceedings 5, no. 1: 62. https://doi.org/10.3390/CSAC2021-10471
APA StyleTorre, R., Freitas, M., Costa-Rama, E., Nouws, H. P. A., & Delerue-Matos, C. (2021). Tropomyosin Analysis in Foods Using an Electrochemical Immunosensing Approach. Chemistry Proceedings, 5(1), 62. https://doi.org/10.3390/CSAC2021-10471









