Electrochemical Detection of Fenthion Insecticide in Olive Oils by a Sensitive Non-Enzymatic Biomimetic Sensor Enhanced with Metal Nanoparticles †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Solutions
2.2. Synthesis of MIP and NIP Materials on the Gold Electrode
2.3. .Electrochemical Measurements
2.4. Analysis of Olive Oil Samples
3. Results and Discussion
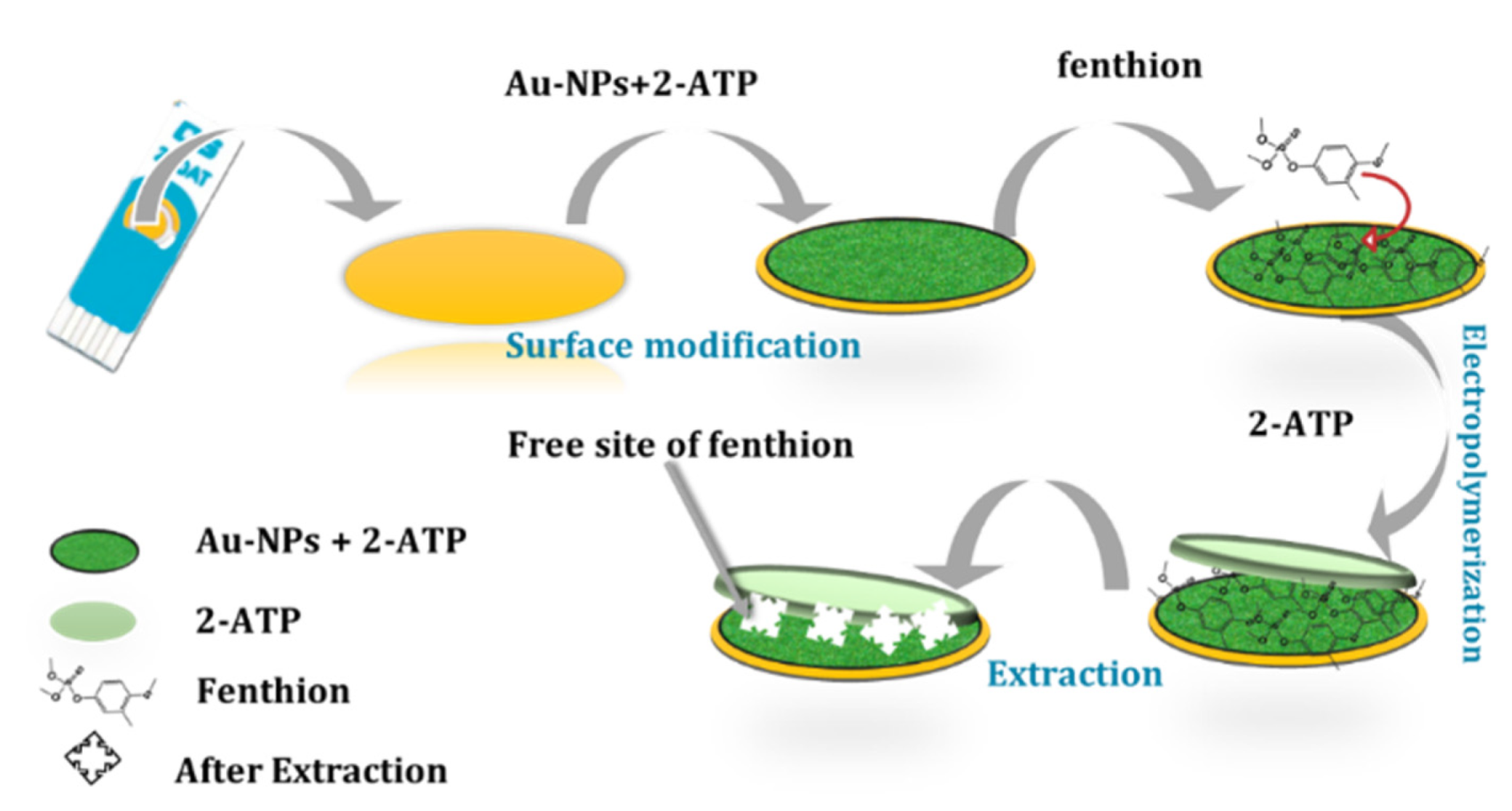
3.1. Electropolymerization of FEN Imprinted Film
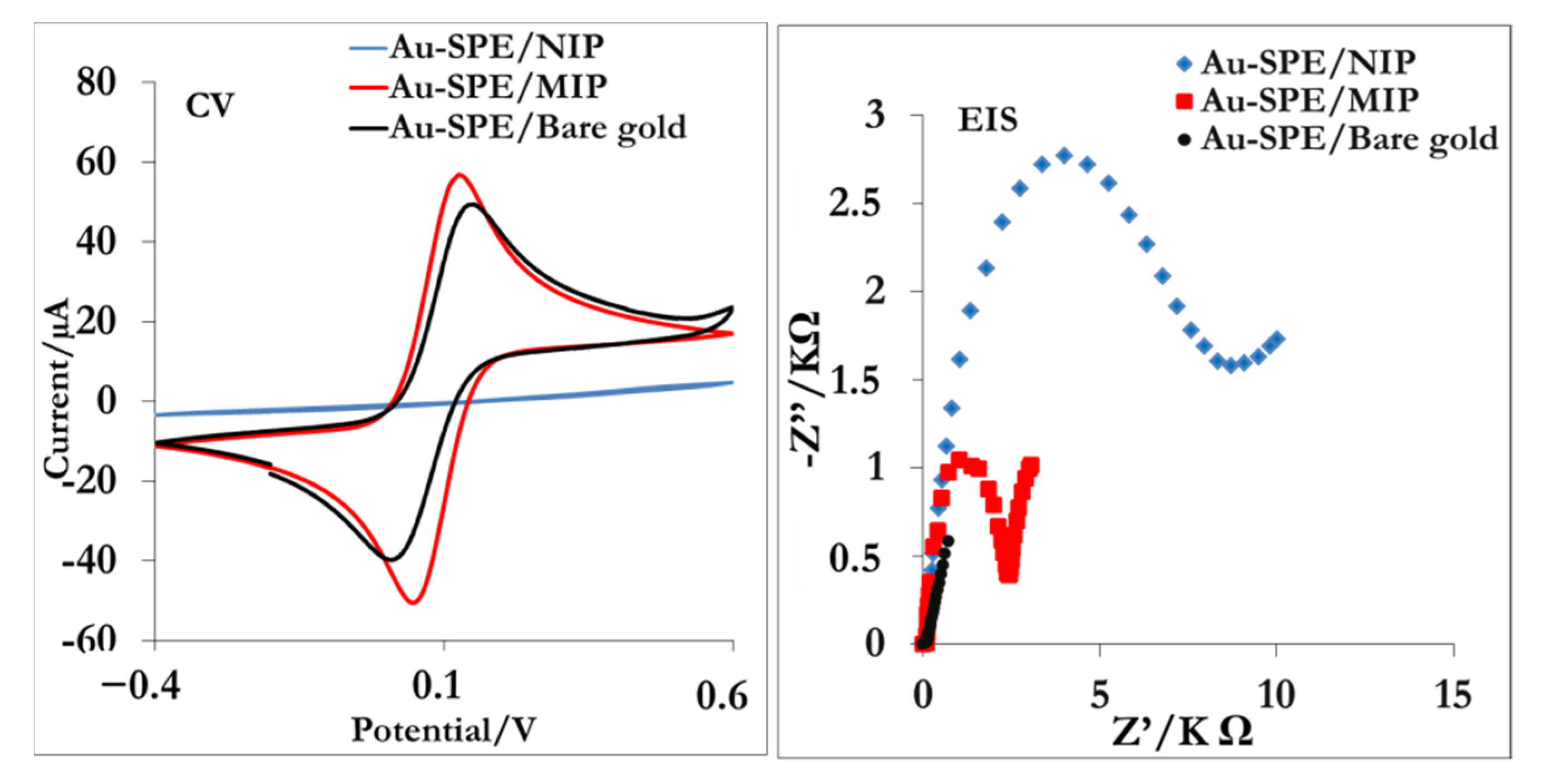
3.2. Molecular Recognition by MIP and NIP Sensors
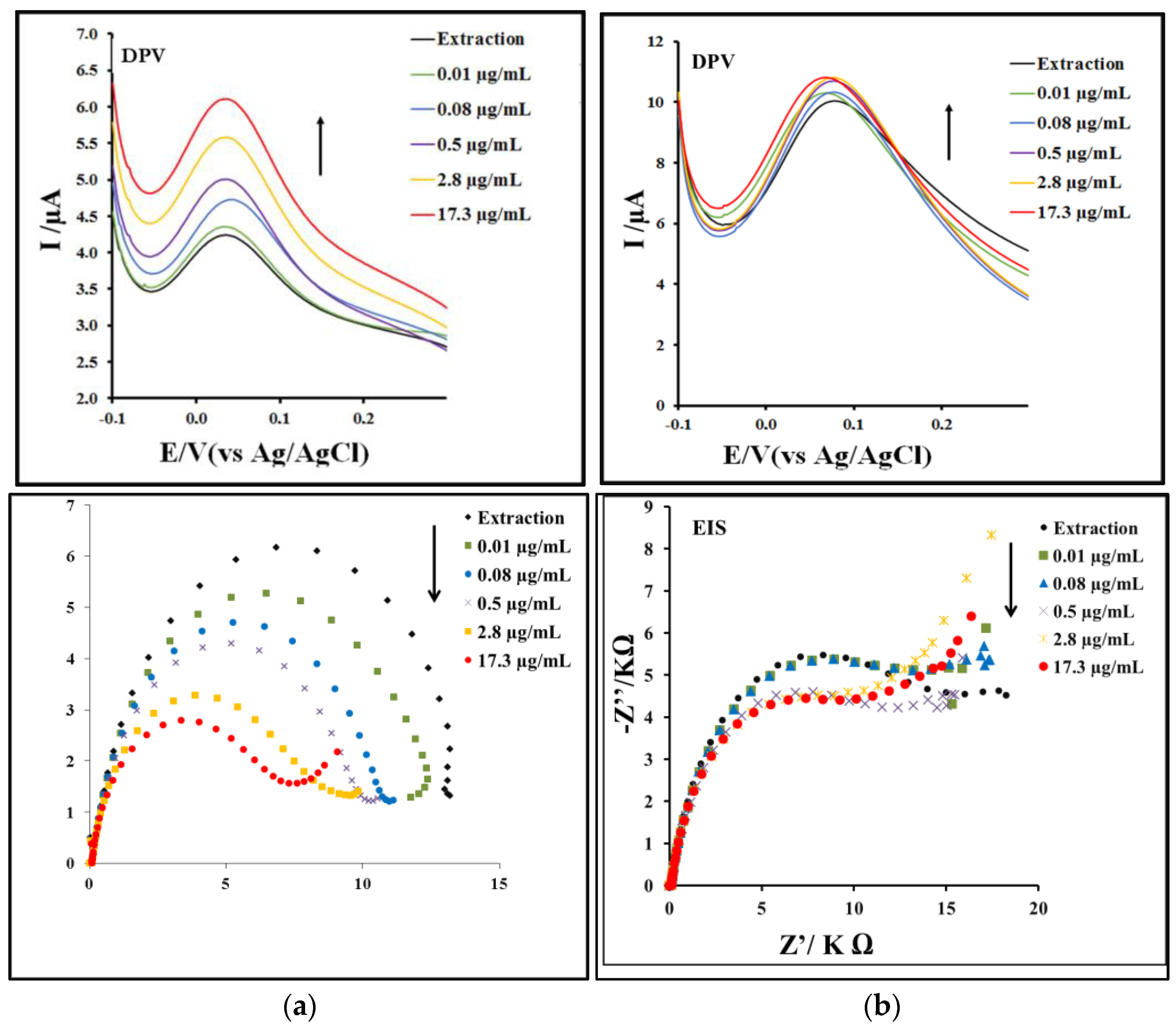
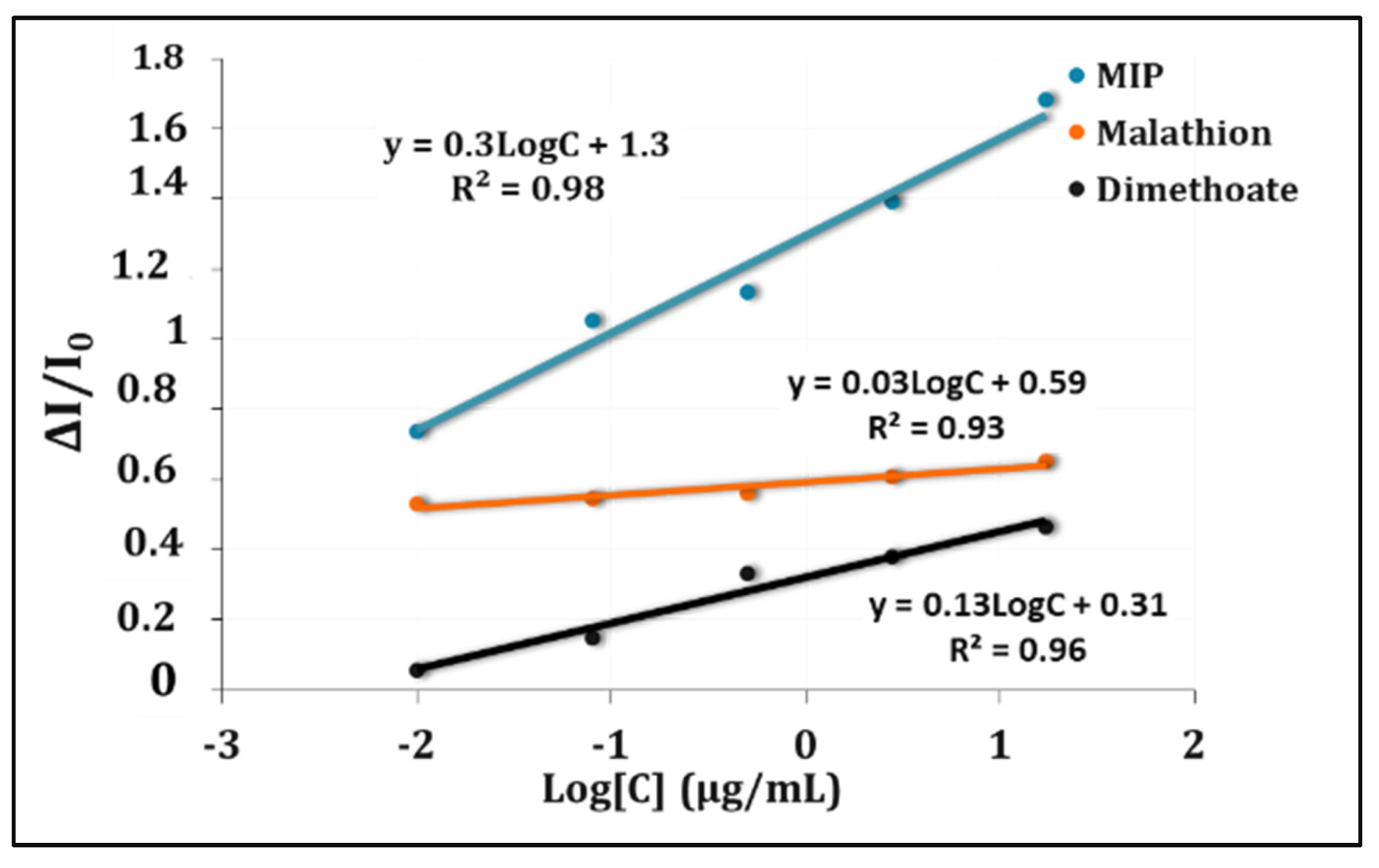
3.3. Calibration Curve and Detection Limit
3.4. Selectivity of the MIP Sensor
3.5. Analysis of Olive Oil Samples
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Garcés-García, M.; Brun, E.M.; Puchades, R.; Maquieira, Á. Immunochemical determination of four organophosphorus insecticide residues in olive oil using a rapid extraction process. Anal. Chim. Acta 2006, 556, 347–354. [Google Scholar] [CrossRef]
- Lentza-Rizos, C. Monitoring pesticide residues in olive products: Organophosphorus insecticides in olives in oil. J. AOAC Int. 1994, 77, 1096–1100. [Google Scholar] [CrossRef]
- Morchio, G.; de Andreis, R.; Verga, G.R. Indagine sul contenuto di composti fosforganici presenti negli oli vegetali e in particolare nell’olio di olive. Riv. Ital. Sostanze Grasse 1992, 69, 147–157. [Google Scholar]
- Hiskia, A.E.; Atmajidou, M.E.; Tsipi, D.F. Determination of organophosphorus pesticide residues in Greek virgin olive oil by capillary gas chromatography. J. Agric. Food Chem. 1998, 46, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, R.; Vazquez, A.; Andini, J.C.; Villén, J. Automated multiresidue analysis of pesticides in olive oil by on-line reversed-phase liquid chromatography–gas chromatography using the through oven transfer adsorption–desorption interface. J. Chromatogr. A 2004, 1029, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Wang, Z.; Wang, J. Determination of Organophosphorus Pesticide Residues by an Acetylcholinesterase Biosensor in Vegetables and Fruits. J. Food Sci. 2007, 28, 229–231. [Google Scholar]
- Zhanga, Q.; Sun, Q.; Hu, B.; Shen, Q.; Yang, G.; Liang, X.; Sun, X.; Liu, F. Development of a sensitive ELISA for the analysis of the organophosphorous insecticide fenthion in fruit samples. Food Chem. 2008, 106, 1278–1284. [Google Scholar] [CrossRef]
- Cho, Y.; Cha, G.S.; Lee, Y.T.; Lee, H.S. A dipstick-type electrochemical immunosensor for the detection of the organophosphorus insecticide fenthion. Food Sci. Biotechnol. 2005, 14, 743–746. [Google Scholar]
- Krämer, P.M.; Franke, A.; Zherdev, A.Z.; Yazynina, E.V.; Dzantiev, B.B. Comparison of two express immunotechniques with polyelectrolyte carriers, ELISA and FIIAA, for the analysis of atrazine. Talanta 2005, 65, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Ghindilis, A.L.; Atanasov, P.; Wilkins, M.; Wilkins, E. Immunosensors: Electrochemical sensing and other engineering approaches. Biosens. Bioelectron. 1998, 13, 113–131. [Google Scholar] [CrossRef]
- Bakas, I.; Oujji, N.B.; Istamboulié, G.; Piletsky, S.; Piletska, E.; Ait-Addi, E.; Ait-Ichou, I.; Noguer, T.; Rouillon, R. Molecularly imprinted polymer cartridges coupled to high performance liquid chromatography (HPLC-UV) for simple and rapid analysis of fenthion in olive oil. Talanta 2014, 125, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Şengül, Ü. Comparing determination methods of detection and quantification limits for aflatoxin analysis in hazelnut. J. Food Drug Anal. 2016, 24, 56–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakas, I.; Oujji, N.B.; Moczkoc, E.; Istambouliea, G.; Piletskyc, S.; Piletskac, E.; Ait-Ichoub, I.; Ait-Addi, E.; Noguer, T.; Rouillona, R. Molecular imprinting solid phase extraction for selective detection of methidathion in olive oil. Anal. Chim. Acta 2012, 734, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Capoferri, D.; Del Carlo, M.; Ntshongontshi, N.; Iwuoha, E.I.; Sergi, M.; Di Ottavio, F.; Compagnone, D. MIP-MEPS based sensing strategy for the selective assay of dimethoate, Application to wheat flour samples. Talanta 2017, 174, 599–604. [Google Scholar] [CrossRef] [PubMed]

| Samples | Concentrations (mg/kg) | RSD (%) (n = 2) |
|---|---|---|
| Commercial oil Al Horra | 0.3125 | 0.14 |
| Ouarzazate field oil | 0.925 | 0.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aghoutane, Y.; Bari, N.E.; Laghrari, Z.; Bouchikhi, B. Electrochemical Detection of Fenthion Insecticide in Olive Oils by a Sensitive Non-Enzymatic Biomimetic Sensor Enhanced with Metal Nanoparticles. Chem. Proc. 2021, 5, 64. https://doi.org/10.3390/CSAC2021-10773
Aghoutane Y, Bari NE, Laghrari Z, Bouchikhi B. Electrochemical Detection of Fenthion Insecticide in Olive Oils by a Sensitive Non-Enzymatic Biomimetic Sensor Enhanced with Metal Nanoparticles. Chemistry Proceedings. 2021; 5(1):64. https://doi.org/10.3390/CSAC2021-10773
Chicago/Turabian StyleAghoutane, Youssra, Nezha El Bari, Zoubida Laghrari, and Benachir Bouchikhi. 2021. "Electrochemical Detection of Fenthion Insecticide in Olive Oils by a Sensitive Non-Enzymatic Biomimetic Sensor Enhanced with Metal Nanoparticles" Chemistry Proceedings 5, no. 1: 64. https://doi.org/10.3390/CSAC2021-10773
APA StyleAghoutane, Y., Bari, N. E., Laghrari, Z., & Bouchikhi, B. (2021). Electrochemical Detection of Fenthion Insecticide in Olive Oils by a Sensitive Non-Enzymatic Biomimetic Sensor Enhanced with Metal Nanoparticles. Chemistry Proceedings, 5(1), 64. https://doi.org/10.3390/CSAC2021-10773







