Abstract
Palladium has wide application in different contexts and, as a consequence, high levels of palladium in the environment have been reported, representing a risk to human health. Considering the interest to develop more selective and sensitive chemosensors for this analyte, two novel benzoxazolyl-alanine derivatives bearing a crown ether moiety were studied as potential fluorimetric chemosensors for palladium detection. Preliminary chemosensory studies for these unnatural amino acids in the presence of selected metal cations were performed in acetonitrile solution and in aqueous mixtures of sodium dodecyl sulfate (SDS, 20 mM, pH 7.5) solution with acetonitrile, 90:10 v/v. In acetonitrile solution, these probes had a fluorescence response for different cations but, most importantly, in SDS aqueous solution both compounds displayed a selective fluorescence response in the presence of palladium.
1. Introduction
Palladium (Pd) is a transition metal extensively applied in several fields due to its special chemical and physical properties. For example, it is used in dental restorations, chemical catalysts, jewelry, electric equipment, automobile industry, among others [1,2]. As a consequence of its wide application, high levels of palladium in the environment have been reported, representing a risk to human health [3,4]. So far, a variety of small fluorescence probes have been successfully developed for Pd2+ detection [5], but there is an interest to design improved water-soluble probes for recognition of this metal in biological and environmental systems [1,6].
For the sensing of metallic cations, there are reports on fluorescent sensors based on amino acids containing different heterocyclic fluorescent and/or coordination units at the side chain [7,8,9,10,11,12,13]. Metal cations are known to be complexed through electron donor atoms at the main/side chains in amino acids, and the insertion of heterocycles at the side chain of natural amino acids yields novel unnatural amino acids with added functionality. In particular, the inclusion of crown ethers is largely used in the design of new chemosensors due to their unique ability to coordinate the cations of alkaline metals and they are also effective complexing reagents for alkaline-earth and transition metal ions [14,15].
Bearing these facts in mind, and considering the research group’s experience on the design, synthesis and characterization of fluorescent chemosensors [7,8,9,10,11,12], we report herein the evaluation of two benzoxazolyl-alanine derivatives bearing a crown ether moiety as potential fluorimetric chemosensors for Pd2+ detection in aqueous media. Preliminary chemosensory studies for these unnatural amino acids in the presence of selected metal cations, with biological and environmental relevance, were performed in acetonitrile solution and in aqueous mixtures of SDS (20 mM, pH 7.5) solution with acetonitrile, 90:10 v/v.
2. Experimental Section
Methods and Materials
The synthesis and structural characterization of benzoxazolyl-alanine derivatives 1a-b has been reported elsewhere [16]. For the photophysical characterization, UV-vis absorption spectra were obtained in acetonitrile solution (1.0 × 10−5 M) using a Shimadzu UV/2501PC spectrophotometer (Shimadzu Europa GmbH, Duisburg, Germany) and the fluorescence spectra were obtained using a Horiba FluoroMax-4 spectrofluorometer (HORIBA Europe GmbH, Darmstadt, Germany), using 9,10-diphenylanthracene in ethanol as fluorescence standard [7].
Evaluation of benzoxazolyl-alanine derivatives 1a-b as fluorimetric chemosensors was carried out in the presence of several cations (Ag+, K+, Li+, Hg2+, Ca2+, Co2+, Pb2+, Mn2+, Fe2+, Zn2+, Ni2+, Cd2+, Cu2+, Pd2+, Fe3+ and Al3+). Solutions of compounds 1a-b (3.0 × 10−5 M) and of the ions under study (1.0 × 10−2 M) were prepared in acetonitrile and acetonitrile/water (75:25). Solutions of the compounds 1a-b (1.0 × 10−5 M) and of the ions under study (1.0 × 10−2 M) were prepared in aqueous mixtures of SDS (20 mM, pH 7.5) solution with acetonitrile, 90:10 v/v. Preliminary studies were carried out by addition of up to 10 equivalents of each cation to the solution of compounds 1a-b in acetonitrile and in mixture of acetonitrile/water. A similar study was performed by addition of up to 10 and 20 equivalents of each cation to the solution of compounds 1a-b in aqueous environments using SDS. The solutions were analyzed in a CN15 viewing cabinet under UV lamp at 365 nm (Vilber Lourmat, Marne-la-Vallée, France).
3. Results and Discussion
Two benzoxazolyl-alanine derivatives bearing a crown ether moiety 1a-b (Figure 1), previously synthesized, were characterized by UV-vis absorption and fluorescence spectroscopy. In both compounds, there is a protected benzoxazolyl-alanine core which is substituted at position 2 of the oxazole ring with a phenyl linked to a 15C5 azacrown ether moiety (1a) or a thiophene coupled to a 18C6 benzocrown ether (1b).
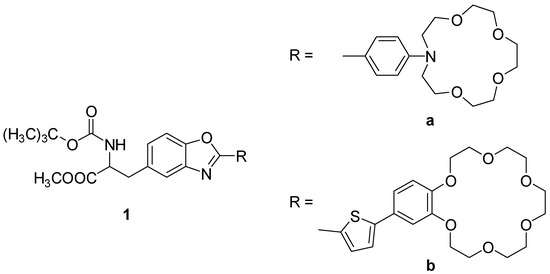
Figure 1.
Crown ether benzoxazolyl-alanine derivatives (1a-b).
Solutions of crown ether benzoxazolyl-alanines 1a-b, in acetonitrile (1.0 × 10−5 M), were analyzed and the wavelengths of maximum absorption and fluorescence, λabs and λflu, molar absorptivities at the absorption maximum, ε, relative fluorescence quantum yields, ΦF, and Stokes’ shifts were compiled in Table 1. Both compounds are highly fluorescent and the higher conjugation in compound 1b is in agreement with the observed bathochromic shift in absorption and fluorescence, when compared to compound 1a.

Table 1.
UV-vis absorption and fluorescence data of crown ether benzoxazolyl-alanines 1a-b, in acetonitrile (1.0 × 10−5 M).
The novel benzoxazolyl-alanines 1a-b were evaluated as fluorimetric chemosensors for the detection of metal cations, with biological and environmental relevance, through preliminary chemosensory studies.
Firstly, the fluorimetric behavior of compounds 1a-b in the presence of selected cations was studied in acetonitrile, by addition of 10 equivalents of each cation. As expected, these probes had a different fluorimetric response for different cations: compound 1a exhibited a remarkable fluorescence quenching upon interaction with Hg2+, Pb2+, Fe2+ and Pd2+ (Figure 2a), whereas compound 1b interacted with Hg2+ and Pd2+ through a decrease of fluorescence and a complete quenching was seen in the presence of Fe3+ (Figure 3a). Considering the importance of water-soluble probes for recognition of metals in biological and environmental systems, the fluorimetric response of compounds 1a-b to selected cations was tested in mixtures of acetonitrile/water (75:25). However, a relevant response was not observed in these conditions.
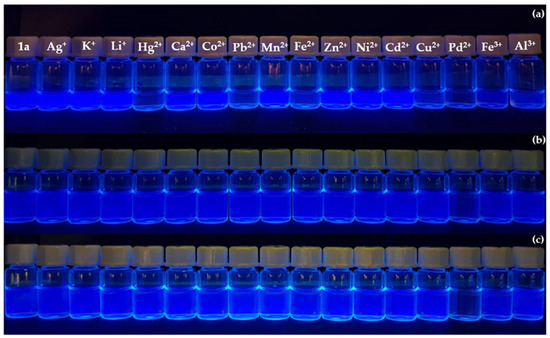
Figure 2.
Preliminary chemosensing study of benzoxazolyl-alanine derivative 1a: (a) in the presence of 10 equivalents of each cation, in acetonitrile (3.0 × 10−5 M); (b) in the presence of 10 equivalents of each cation, in SDS (20 mM, pH 7.5)-acetonitrile 90:10 (v/v) solution (1.0 × 10−5 M); (c) in the presence of 20 equivalents of each cation, in SDS (20 mM, pH 7.5)-acetonitrile 90:10 (v/v) solution (1.0 × 10−5 M).

Figure 3.
Preliminary chemosensing study of benzoxazolyl-alanine derivative 1b: (a) in the presence of 10 equivalents of each cation, in acetonitrile (3.0 × 10−5 M); (b) in the presence of 10 equivalents of each cation, in SDS (20 mM, pH 7.5)-acetonitrile 90:10 (v/v) solution (1.0 × 10−5 M); (c) in the presence of 20 equivalents of each cation, in SDS (20 mM, pH 7.5)-acetonitrile 90:10 (v/v) solution (1.0 × 10−5 M).
Based on our previous experience, the use of an anionic surfactant such as sodium dodecylsulfate (SDS) was attempted to overcome this problem [17]. In fact, several authors reported that in aqueous environments using surfactants, selected binding sites and fluorophores can be arranged in micelles of surfactants allowing detection of metal cations in water by changes in fluorescence [18,19]. Taking this into account, solutions of compounds 1a-b were prepared in aqueous mixtures of SDS (20 mM, pH 7.5) solution with acetonitrile, 90:10 v/v. SDS aqueous solutions of probes 1a-b displayed a selective fluorescence quenching in the presence of 10 equivalents of Pd2+ (Figure 2b and Figure 3b). Furthermore, further addition to 20 equivalents of each ion confirmed the selectivity of both crown ether benzoxazolyl-alanine derivatives 1a-b for Pd2+ (Figure 2c and Figure 3c).
4. Conclusions
In summary, two novel benzoxazolyl-alanines bearing a crown ether moiety 1a-b were evaluated as fluorimetric chemosensors for several ions in acetonitrile and in mixtures of acetonitrile and aqueous SDS solution. As expected, these probes had a fluorimetric response for different cations in acetonitrile solutions but, most importantly, in aqueous mixtures using SDS anionic surfactant both crown ether benzoxazolyl-alanines displayed a selective fluorimetric quenching in the presence of Pd2+. These results clearly indicated that probes 1a-b could be used to detect the palladium cation in environmental and biological samples, with remarkable selectivity.
Acknowledgments
The authors acknowledge Fundação para a Ciência e Tecnologia—FCT (Portugal) for funding through CQUM (UIDB/00686/2020).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sun:, Q.; Qiu, Y.; Chen, J.; Wu, F.S.; Luo, X.G.; Guo, Y.R.; Han, X.Y.; Wang, D.W. A Colorimetric and Fluorescence Turn-on Probe for the Detection of Palladium in Aqueous Solution and its Application in vitro and in vivo. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 239, 118547. [Google Scholar] [CrossRef] [PubMed]
- Ke, B.; Chen, H.; Cui, Y.; Ma, L.; Liu, Y.; Hu, X.; Bai, Y.; Du, L.; Li, M. A Bioluminescent Strategy for Imaging Palladium in Living Cells and Animals with Chemoselective Probes Based on Luciferin-Luciferase System. Talanta 2019, 194, 925–929. [Google Scholar] [CrossRef] [PubMed]
- Iavicoli, I.; Fontana, L.; Bergamaschi, A. Palladium: Exposure, Uses, and Human Health Effects. In Encyclopedia of Environmental Health, 1st ed.; Nriagu, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 307–314. [Google Scholar] [CrossRef]
- Kielhorn, J.; Melber, C.; Keller, D.; Mangelsdorf, I. Palladium—A Review of Exposure and Effects to Human Health. Int. J. Hyg. Environ. Health 2002, 205, 417–432. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, R.; Liu, J.H.; Liu, B.T. A Review of Recent Developments in Fluorescent Sensors for the Selective Detection of Palladium Ions. Coord. Chem. Rev. 2018, 376, 196–224. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, L.; Zhou, Y.; Ma, T.; Niu, J. A Highly Selective Fluorescent Probe for the Detection of Palladium(II) Ion in Cells and Aqueous Media. Microchim. Acta 2013, 180, 211–217. [Google Scholar] [CrossRef]
- Ferreira, R.C.M.; Raposo, M.M.M.; Costa, S.P.G. Heterocyclic Amino Acids as Fluorescent Reporters for Transition Metals: Synthesis and Evaluation of Novel Furyl-benzoxazol-5-yl-L-alanines. New J. Chem. 2018, 42, 3483–3492. [Google Scholar] [CrossRef]
- Esteves, C.I.C.; Ferreira, R.C.M.; Raposo, M.M.M.; Costa, S.P.G. New Fluoroionophores for Metal Cations Based on Benzo[d]oxazol-5-yl-alanine Bearing Pyrrole and Imidazole. Dyes Pigments 2018, 151, 211–218. [Google Scholar] [CrossRef]
- Esteves, C.I.C.; Raposo, M.M.M.; Costa, S.P.G. New 2,4,5-Triarylimidazoles Based on a Phenylalanine Core: Synthesis, Photophysical Characterization and Evaluation as Fluorimetric Chemosensors for Ion Recognition. Dyes Pigments 2016, 134, 258–268. [Google Scholar] [CrossRef]
- Esteves, C.I.C.; Raposo, M.M.M.; Costa, S.P.G. Synthesis and Evaluation of Benzothiazolyl and Benzimidazolyl Asparagines as Amino Acid Based Selective Fluorimetric Chemosensors for Cu2+. Tetrahedron 2010, 66, 7479–7486. [Google Scholar] [CrossRef]
- Martins, C.D.F.; Raposo, M.M.M.; Costa, S.P.G. Metallic Ion Sensing with a Benzothiazole-Based Fluorimetric Chemosensor. Proceedings 2019, 9, 11. [Google Scholar] [CrossRef]
- Esteves, C.I.C.; Batista, R.M.F.; Raposo, M.M.M.; Costa, S.P.G. Novel Functionalised Imidazo-Benzocrown Ethers Bearing a Thiophene Spacer as Fluorimetric Chemosensors for Metal Ion Detection. Dyes Pigments 2016, 135, 134–142. [Google Scholar] [CrossRef]
- Guzow, K.; Szmigiel, D.; Wróblewski, D.; Milewska, M.; Karolczak, J.; Wiczk, W. New Fluorescent Probes Based on 3-(2-Benzoxazol-5-yl)alanine Skeleton—Synthesis and Photophysical Properties. J. Photochem. Photobiol. A 2007, 187, 87–96. [Google Scholar] [CrossRef]
- Fery-Forgues, S.; Al-Ali, F. Bis(Azacrown Ether) and Bis(Benzocrown Ether) Dyes: Butterflies, Tweezers and Rods in Cation Binding. J. Photochem. Photobiol. C 2004, 5, 139–153. [Google Scholar] [CrossRef]
- Gokel, G.W.; Leevy, W.M.; Weber, M.E. Crown Ethers: Sensors for Ions and Molecular Scaffolds for Materials and Biological Models. Chem. Rev. 2004, 104, 2723–2750. [Google Scholar] [CrossRef] [PubMed]
- Batista, P.M.R. Synthesis and Evaluation of Novel Amino Acids Functionalized with Crown ether and Benzoxazole Units as Fluorimetric Sensors for Cations. Master’s Thesis, University of Minho, Braga, Portugal, 2011. [Google Scholar]
- Okda, H.E.; El Sayed, S.; Ferreira, R.C.M.; Gonçalves, R.C.R.; Costa, S.P.G.; Raposo, M.M.M.; Martínez-Máñez, R.; Sancenón, F. N,N-Diphenylanilino-Heterocyclic Aldehyde-Based Chemosensors for UV-Vis/NIR and Fluorescence Cu(II) Detection. New J. Chem. 2019, 43, 7393–7402. [Google Scholar] [CrossRef]
- Pallavicini, P.; Pasotti, L.; Patroni, S. Residual and Exploitable Fluorescence in Micellar Self-Assembled ON–OFF Sensors for Copper(II). Dalton Trans. 2007, 48, 5670–5677. [Google Scholar] [CrossRef] [PubMed]
- Arduini, M.; Rampazzo, E.; Mancin, F.; Tecilla, P.; Tonellato, U. Template Assisted Self-Organized Chemosensors. Inorg. Chim. Acta 2007, 360, 721–727. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).