Oxazole-Based Compounds: Synthesis and Anti-Inflammatory Studies †
Abstract
1. Introduction
2. Results and Discussion
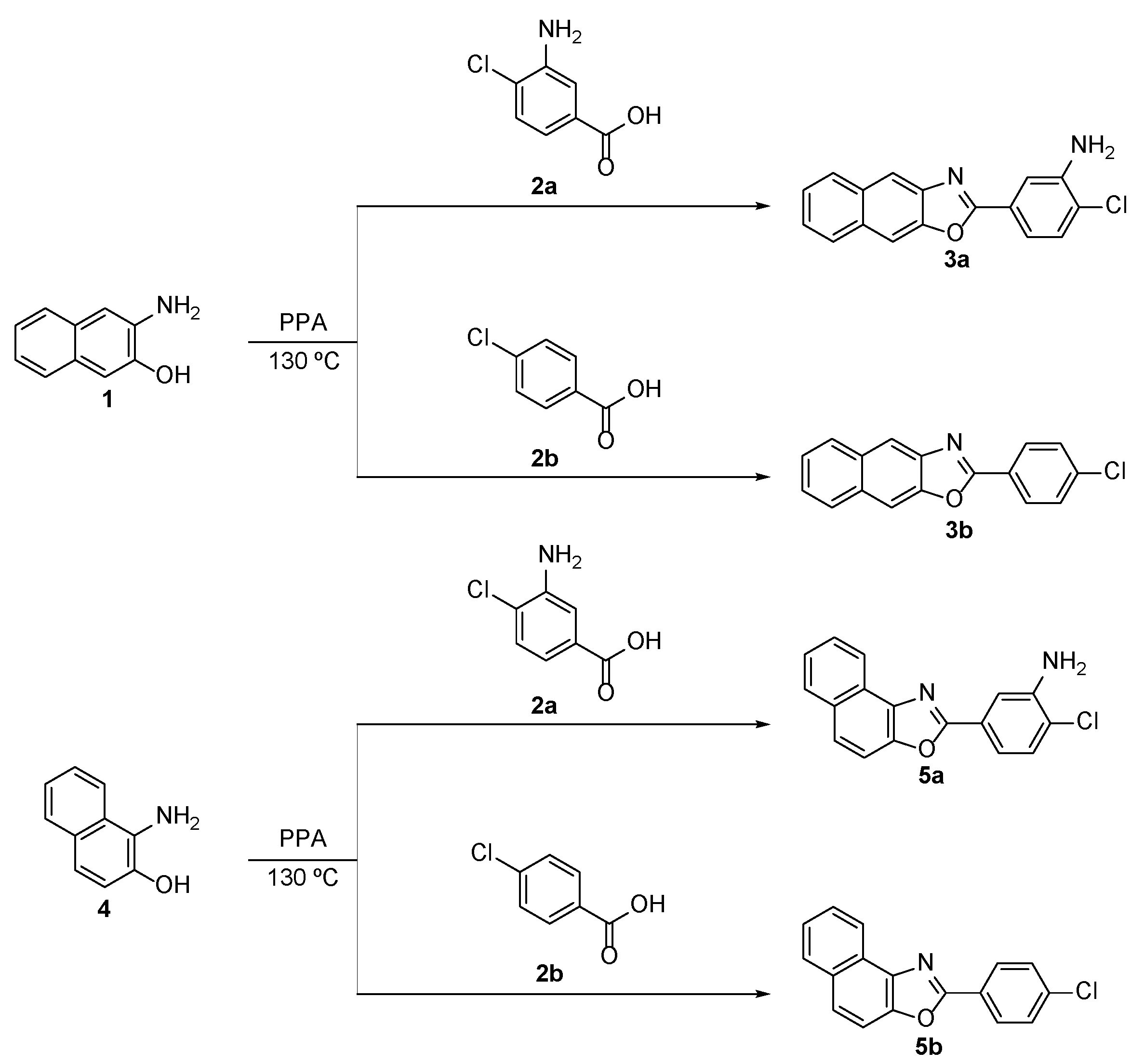
2.1. Synthesis of Naphthoxazole Derivatives
2.2. LOX Inhibition
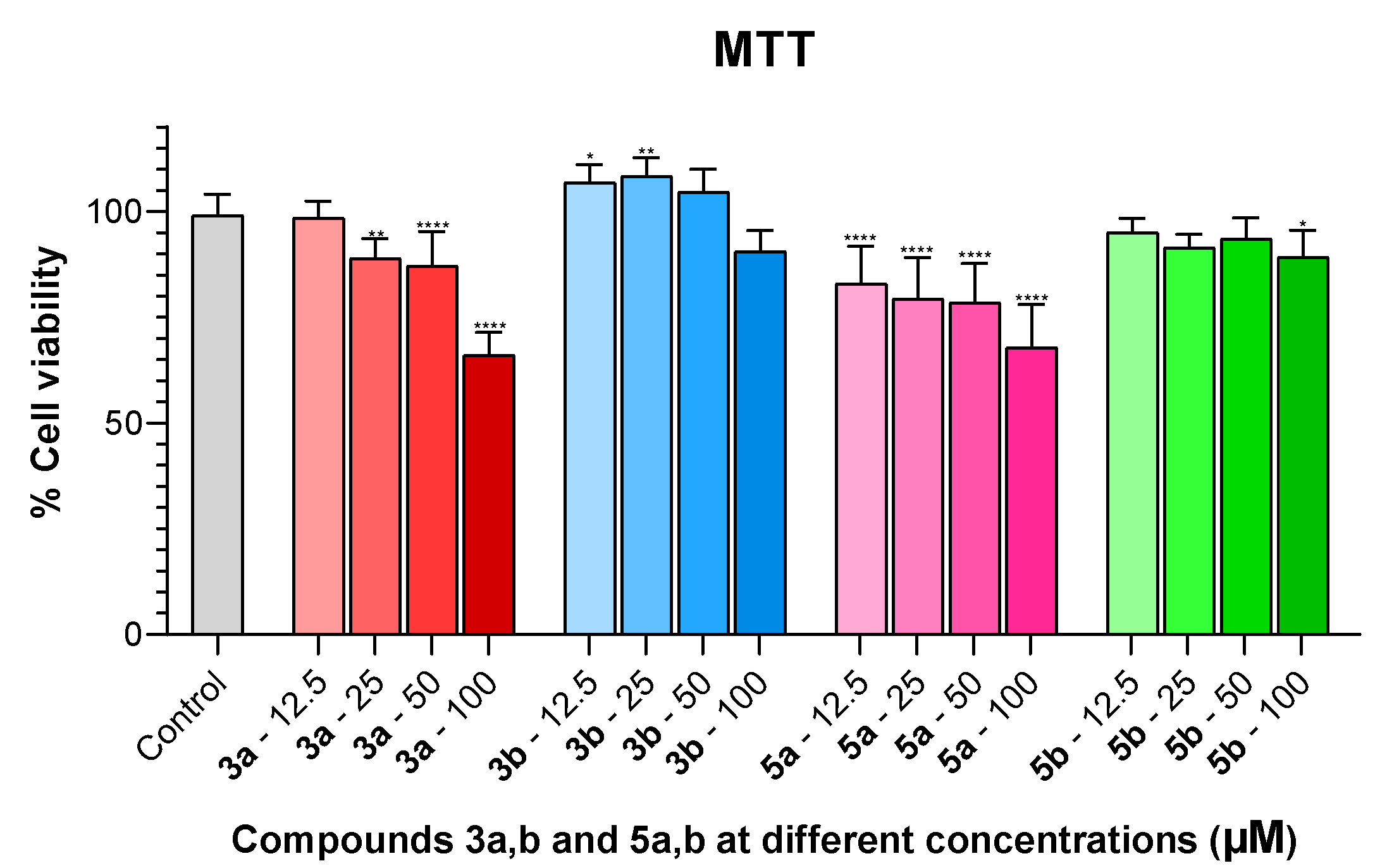
2.3. Viability Assays
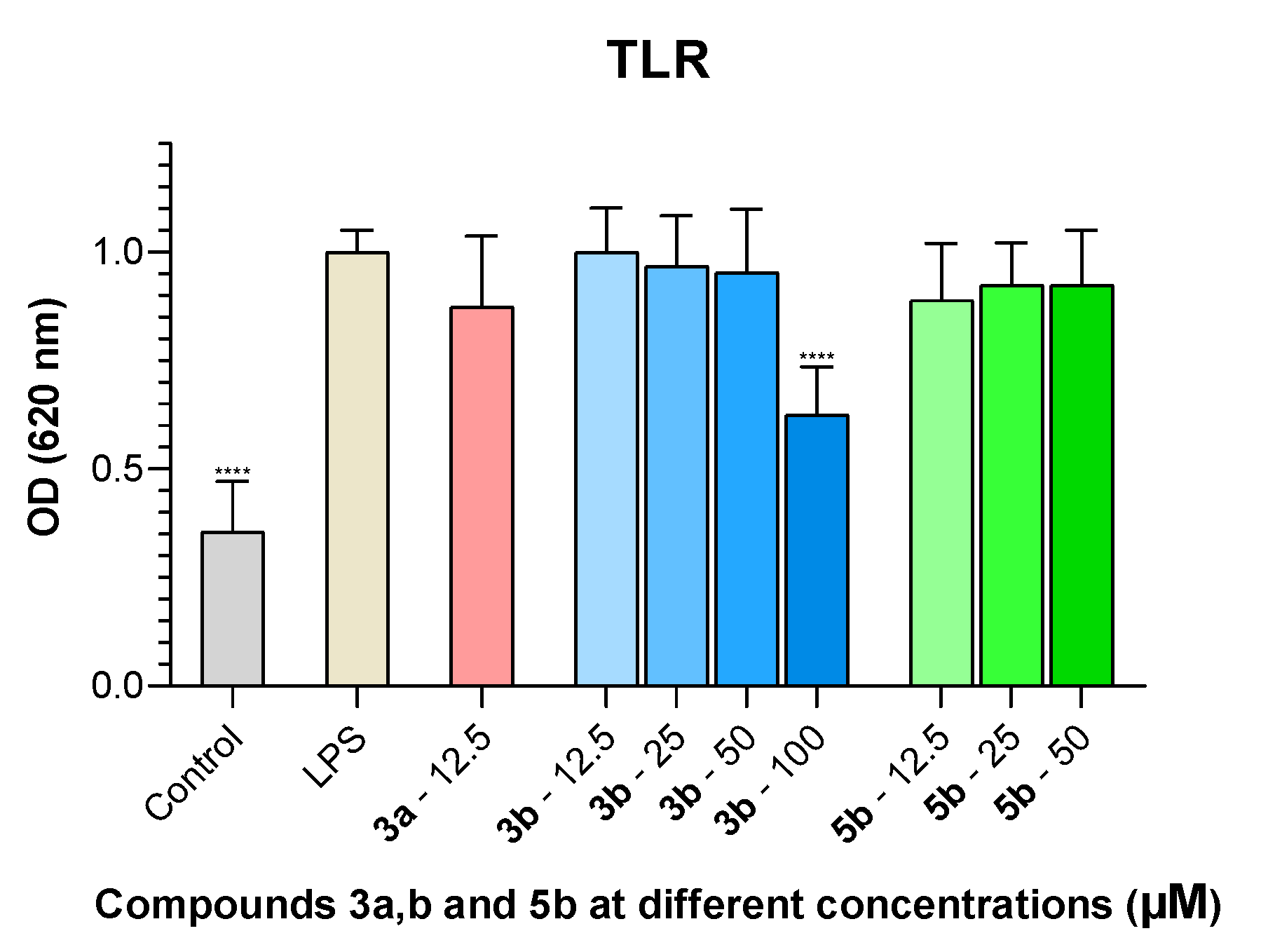
2.4. NF-kB Inhibition
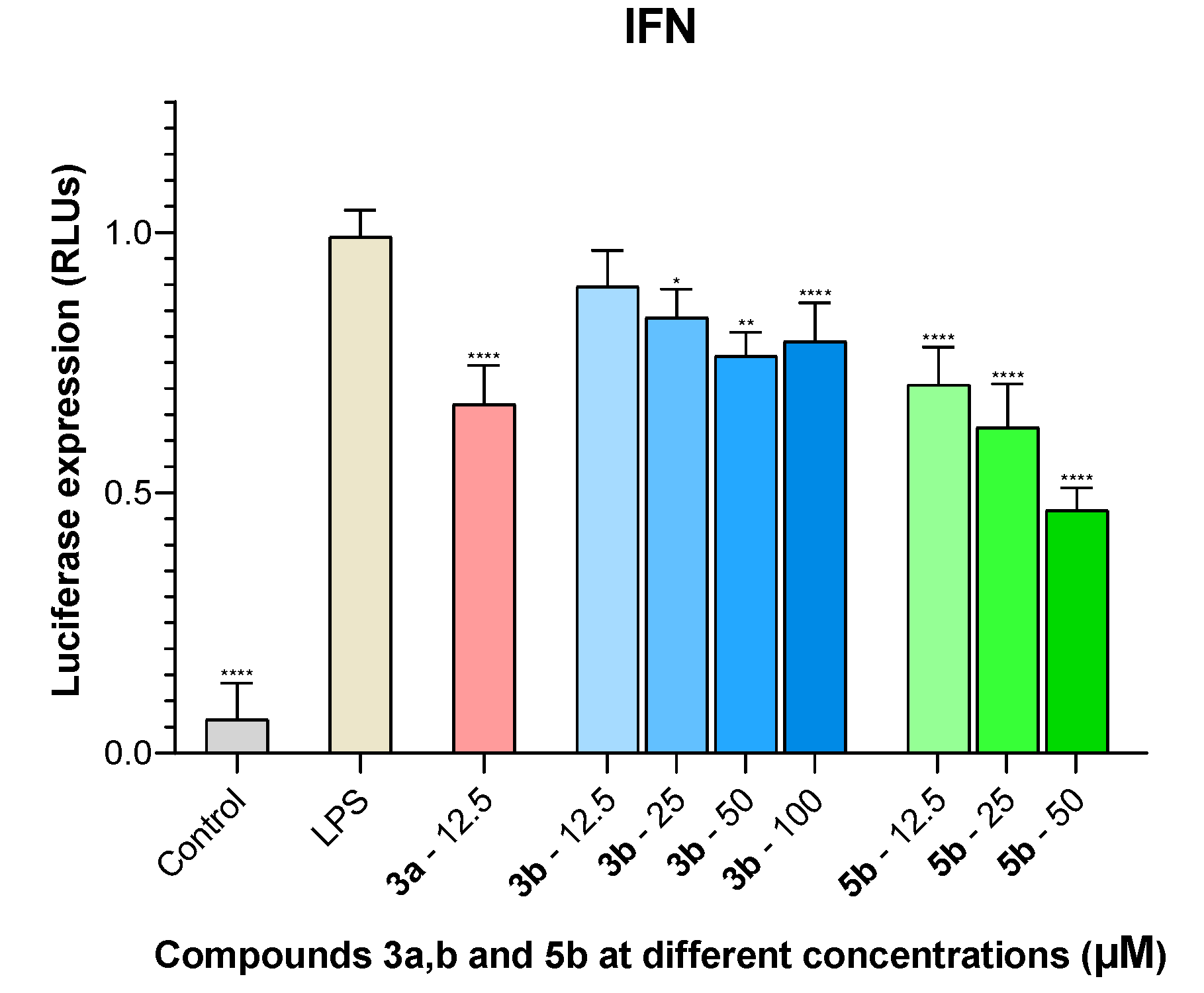
2.5. IFN Inhibition
3. Materials and Methods
3.1. General Procedure for the Preparation of Naphthoxazole Derivatives 3a,b and 5a,b (Illustrated for 5b)
3.2. Biological Assays
3.2.1. Statistical Analysis
3.2.2. LOX Inhibition Procedure
3.2.3. Cell Culture Conditions
3.2.4. MTT Reduction Assays
3.2.5. TLR Stimulation Assay
3.2.6. IFN Induction Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| COX-2 | Cyclooxygenase-2 |
| IFN | Interferon |
| IRF | Interferon Regulatory Factor |
| LOX | Lipoxygenase |
| LPS | Lipopolysaccharide |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NF-kB | Nuclear Factor kappa B |
| NSAIDs | Nonsteroidal Anti-inflammatory Drugs |
| PMA | Phorbol 12-myristate 13-acetate |
| PPA | Polyphosphoric Acid |
| SEAP | Secreted Embryonic Alkaline Phosphatase |
| TLR | Toll-Like Receptor |
References
- Jacob, P.J.; Manju, S.L.; Ethiraj, K.R.; Elias, G. Safer anti-inflammatory therapy through dual COX-2/5-LOX inhibitors: A structure-based approach. Eur. J. Pharm. Sci. 2018, 121, 356–381. [Google Scholar] [CrossRef]
- Megha, K.B.; Joseph, X.; Akhil, V.; Mohanan, P.V. Cascade of immune mechanism and consequences of inflammatory disorders. Phytomedicine 2021, 91, 153712. [Google Scholar] [CrossRef] [PubMed]
- Soares, C.L.R.; Wilairatana, P.; Silva, L.R.; Moreira, P.S.; Barbosa, N.M.M.V.; da Silva, P.R.; Coutinho, H.D.M.; de Menezes, I.R.A.; Felipe, C.F.B. Biochemical aspects of the inflammatory process: A narrative review. Biomed. Pharmacother. 2023, 168, 115764. [Google Scholar] [CrossRef]
- Mukhopadhyay, N.; Shukla, A.; Makhal, P.N.; Kaki, V.R. Natural product-driven dual COX-LOX inhibitors: Overview of recent studies on the development of novel anti-inflammatory agents. Heliyon 2023, 9, e14569. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Peng, X.; Liu, F.; Zhang, Q.; Ding, L.; Li, G.; Qiu, F. Potential of natural products in inflammation: Biological activities, structure–activity relationships, and mechanistic targets. Arch. Pharm. Res. 2024, 47, 377–409. [Google Scholar] [CrossRef] [PubMed]
- Gouda, N.A.; Alshammari, S.O.; Abourehab, M.A.S.; Alshammari, Q.A.; Elkamhawy, A. Therapeutic potential of natural products in inflammation: Underlying molecular mechanisms, clinical outcomes, technological advances, and future perspectives. Inflammopharmacology 2023, 31, 2857–2883. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Mei, Y.; Jiang, L.; Yang, X.; Zeng, W.; Du, Y. Oxazole and isoxazole-containing pharmaceuticals: Targets, pharmacological activities, and their SAR studies. RSC Med. Chem. 2025, 16, 1879–1890. [Google Scholar] [CrossRef] [PubMed]
- Wong, X.K.; Yeong, K.Y. A Patent Review on the Current Developments of Benzoxazoles in Drug Discovery. ChemMedChem 2021, 16, 3237–3262. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, S.; De Rosa, M. The Benzoxazole Heterocycle: A Comprehensive Review of the Most Recent Medicinal Chemistry Developments of Antiproliferative, Brain-Penetrant, and Anti-inflammatory Agents. Top. Curr. Chem. 2024, 382, 33. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lv, S.; Liu, J.; Yu, Y.; Wang, H.; Zhang, H. An Overview of Bioactive 1,3-Oxazole-Containing Alkaloids from Marine Organisms. Pharmaceuticals 2021, 14, 1274. [Google Scholar] [CrossRef] [PubMed]
- Skrzypek, A.; Karpińska, M.; Juszczak, M.; Grabarska, A.; Wietrzyk, J.; Krajewska-Kułak, E.; Studziński, M.; Paszko, T.; Matysiak, J. Cholinesterases Inhibition, Anticancer and Antioxidant Activity of Novel Benzoxazole and Naphthoxazole Analogs. Molecules 2022, 27, 8511. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xiao, X.; Xue, Y.; Zhou, H.; Huang, C.; Zhu, M.; Zhuang, T.; Chen, Y.; Huang, L. Discovery of a novel class of benzoxazole derivatives as histamine H3 receptor ligands for the treatment of neuropathic pain. Bioorg. Chem. 2022, 127, 106039. [Google Scholar] [CrossRef] [PubMed]
- Chiacchio, M.; Lanza, G.; Chiacchio, U.; Giofrè, S.V.; Romeo, R.; Iannazzo, D.; Legnani, L. Oxazole-based compounds as anticancer agents. Curr. Med. Chem. 2020, 26, 7337–7371. [Google Scholar] [CrossRef] [PubMed]
- Apostol, T.; Marutescu, L.G.; Draghici, C.; Socea, L.-I.; Olaru, O.T.; Nitulescu, G.M.; Pahontu, E.M.; Saramet, G.; Enache-Preoteasa, C.; Barbuceanu, S.-F. Synthesis and Biological Evaluation of New N-Acyl-α-amino Ketones and 1,3-Oxazoles Derivatives. Molecules 2021, 26, 5019. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Li, D.; Zhang, A.-L.; Gao, J.-M. Synthesis, Antifungal Activities and Molecular Docking Studies of Benzoxazole and Benzothiazole Derivatives. Molecules 2018, 23, 2457. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-X.; Shi, S.-J.; Long, L.-P.; He, X.-P.; Zhang, P.; Yang, X.-W.; Liu, Y.; Niu, X.-M.; Guo, K.; Li, S.-H. Anti-inflammatory oxazole-, nitro- and hexahydropyrrolo[2,1-b]oxazole-containing abietane diterpenoid alkaloids from Salvia miltiorrhiza. Phytochemistry 2024, 229, 114300. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Liu, W.; Zhang, D. Recent Advances in the Synthesis of Oxazole-Based Molecules via van Leusen Oxazole Synthesis. Molecules 2020, 25, 1594. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, Z.; Liu, F.; Sun, Q.; Li, J. Synthesis and properties of poly(imide-benzoxazole) fibers from 4,40 -oxydiphthalic dianhydride in polyphosphoric acid. Eur. Polym. J. 2015, 64, 108. [Google Scholar] [CrossRef]
- Nawaz, Z.; Riaz, N.; Saleem, M.; Iqbal, A.; Ejaz, S.A.; Muzaffar, S.; Bashir, B.; Ashraf, M.; Rehman, A.-U.; Bilal, M.S.; et al. Probing N-substituted 4-(5-mercapto-4-ethyl-4H-1,2,4-triazol-3-yl)-N-phenylpiperdine-1-carboxamides as potent 15-LOX inhibitors supported with ADME, DFT calculations and molecular docking studies. Heliyon 2024, 10, e35278. [Google Scholar] [CrossRef] [PubMed]
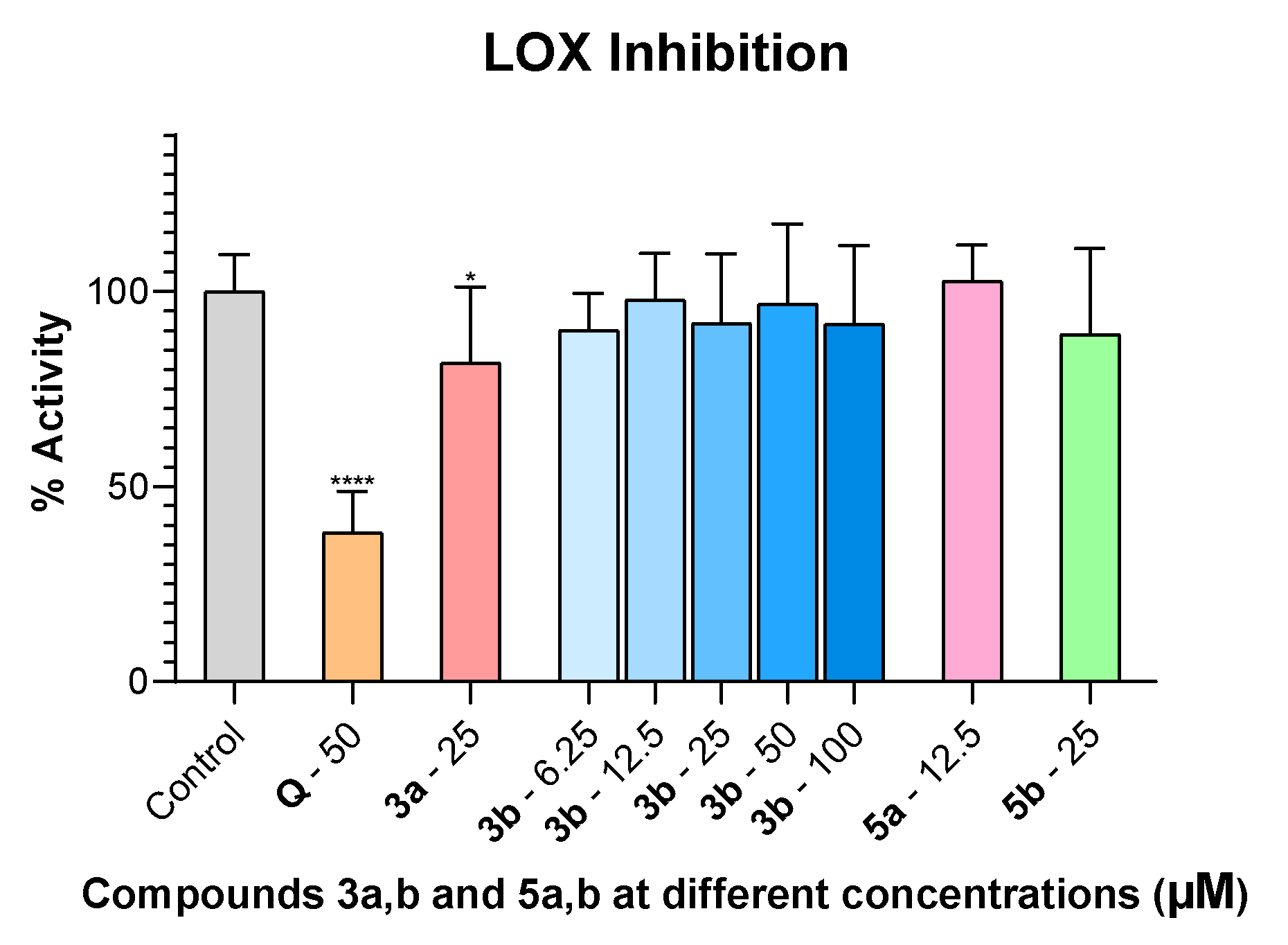
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, S.; Correia da Silva, D.; Pereira, D.M.; Gonçalves, M.S.T. Oxazole-Based Compounds: Synthesis and Anti-Inflammatory Studies. Chem. Proc. 2025, 18, 36. https://doi.org/10.3390/ecsoc-29-26668
Gomes S, Correia da Silva D, Pereira DM, Gonçalves MST. Oxazole-Based Compounds: Synthesis and Anti-Inflammatory Studies. Chemistry Proceedings. 2025; 18(1):36. https://doi.org/10.3390/ecsoc-29-26668
Chicago/Turabian StyleGomes, Sofia, Daniela Correia da Silva, David M. Pereira, and M. Sameiro T. Gonçalves. 2025. "Oxazole-Based Compounds: Synthesis and Anti-Inflammatory Studies" Chemistry Proceedings 18, no. 1: 36. https://doi.org/10.3390/ecsoc-29-26668
APA StyleGomes, S., Correia da Silva, D., Pereira, D. M., & Gonçalves, M. S. T. (2025). Oxazole-Based Compounds: Synthesis and Anti-Inflammatory Studies. Chemistry Proceedings, 18(1), 36. https://doi.org/10.3390/ecsoc-29-26668







