A Computational Study to Determine Thermodynamic Properties for Hydrogen Production from Sodium Borohydride Reaction †
Abstract
1. Introduction
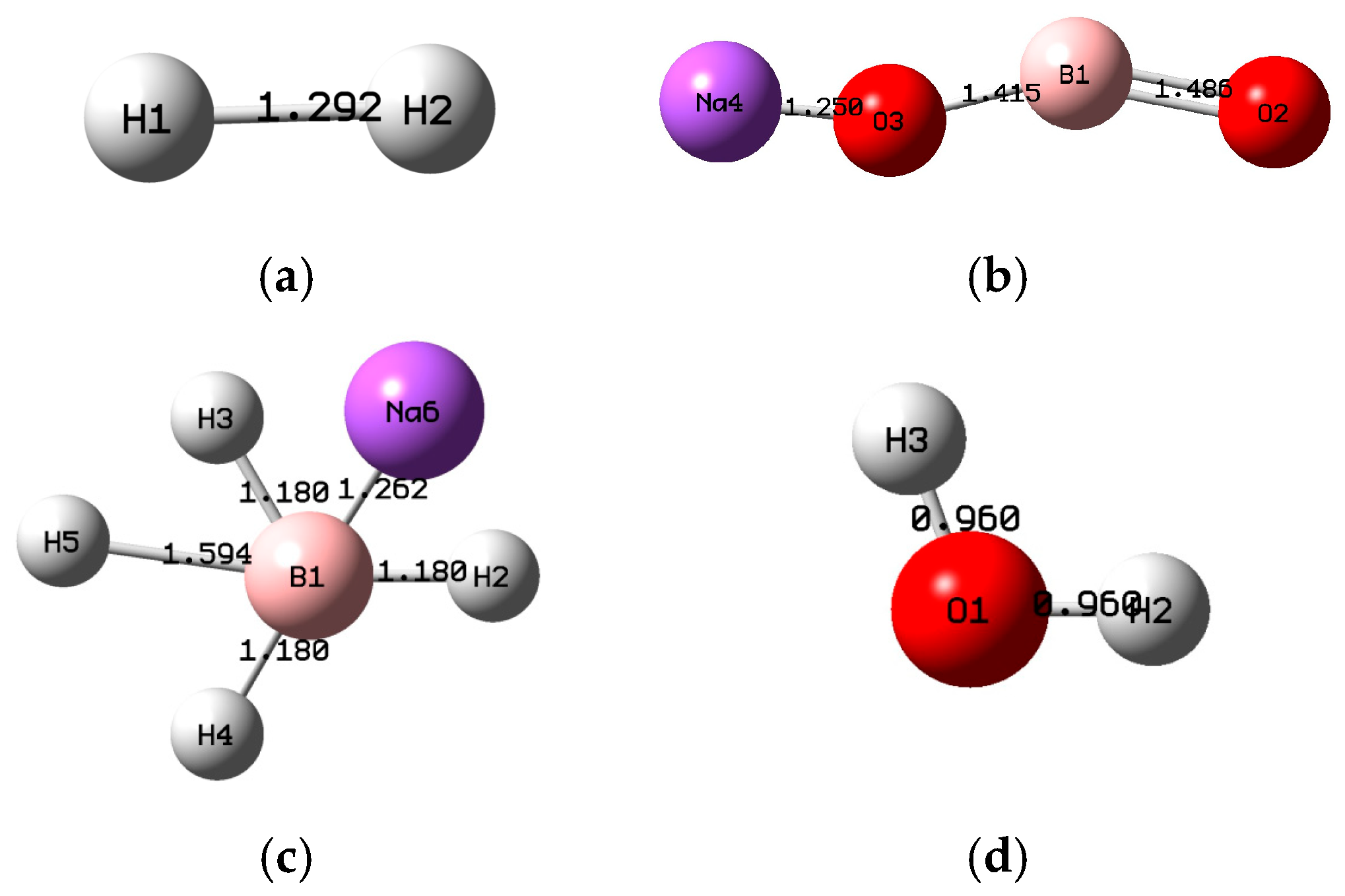
2. Computational Method
3. Results of the Thermodynamic Study
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rivarolo, M.; Improta, O.; Magistri, L.; Panizza, M.; Barbucci, A. Thermo-economic analysis of a hydrogen production system by sodium borohydride (NaBH4). Int. J. Hydrogen Energy 2018, 43, 1606–1614. [Google Scholar] [CrossRef]
- Mirshafiee, F.; Rezaei, M. Engineering of the ferrite-based support for enhanced performance of supported Pt, Pd, Ru, and Rh catalysts in hydrogen generation from NaBH4 hydrolysis. Sci. Rep. 2024, 14, 20818. [Google Scholar] [CrossRef] [PubMed]
- Demirel, E.; Ayas, N. Thermodynamic modeling of the water-gas shift reaction in supercritical water for hydrogen production. Theor. Found. Chem. Eng. 2017, 51, 76–87. [Google Scholar] [CrossRef]
- Nazarova, G.; Ivanchina, E.; Ivashkina, E.; Kiseleva, S.; Stebeneva, V. Thermodynamic analysis of catalytic cracking reactions as the first stage in the development of mathematical description. Procedia Chem. 2015, 15, 342–349. [Google Scholar] [CrossRef]
- Dragan, M. Hydrogen storage in complex metal hydrides NaBH4: Hydrolysis reaction and experimental strategies. Catalysts 2022, 12, 356. [Google Scholar] [CrossRef]
- Hydrogen Tools Portal. Available online: https://h2tools.org/basic-hydrogen-properties-chart (accessed on 1 September 2025).
| NaBH4 | H2O | NaBO2 | H2 | Na | B | H | O | |
|---|---|---|---|---|---|---|---|---|
| ε0 | −187.976351 | −76.009862 | −335.860216 | −1.037482 | −161.841435 | −24.522037 | −0.498233 | −74.656604 |
| εZPE | 0.050072 | 0.022129 | 0.009946 | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 0.000000 |
| Etot | 0.053187 | 0.024963 | 0.013374 | 0.002360 | 0.001416 | 0.001416 | 0.001416 | 0.001416 |
| Hcorr | 0.054131 | 0.025907 | 0.014318 | 0.003305 | 0.002360 | 0.002360 | 0.002360 | 0.002360 |
| Gcorr | 0.027274 | 0.004514 | −0.015115 | −0.012532 | −0.015083 | −0.014040 | −0.010654 | −0.013915 |
| ε0 + εZPE | −187.926279 | −75.987733 | −335.850270 | −1.037482 | −161.841435 | −24.522037 | −0.498233 | −74.656604 |
| ε0 + Etot | −187.923163 | −75.984899 | −335.846842 | −1.035121 | −161.840019 | −24.520621 | −0.496817 | −74.655188 |
| ε0 + Hcorr | −187.922219 | −75.983954 | −335.845898 | −1.034177 | −161.839075 | −24.519677 | −0.495872 | −74.654244 |
| ε0 + Gcorr | −187.949077 | −76.005348 | −335.875331 | −1.050014 | −161.856518 | −24.536078 | −0.508887 | −74.670519 |
| Thermodynamic Property | Unit | Compound | |||
|---|---|---|---|---|---|
| NaBH4 | H2O | NaBO2 | H2 | ||
| Entropy (S) | Cal/mol·K | 56.526 | 45.027 | 61.947 | 33.331 |
| Heat Capacity (Cv) | 8.052 | 5.986 | 9.134 | 4.968 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Özçakır, G. A Computational Study to Determine Thermodynamic Properties for Hydrogen Production from Sodium Borohydride Reaction. Chem. Proc. 2025, 18, 39. https://doi.org/10.3390/ecsoc-29-26733
Özçakır G. A Computational Study to Determine Thermodynamic Properties for Hydrogen Production from Sodium Borohydride Reaction. Chemistry Proceedings. 2025; 18(1):39. https://doi.org/10.3390/ecsoc-29-26733
Chicago/Turabian StyleÖzçakır, Gamze. 2025. "A Computational Study to Determine Thermodynamic Properties for Hydrogen Production from Sodium Borohydride Reaction" Chemistry Proceedings 18, no. 1: 39. https://doi.org/10.3390/ecsoc-29-26733
APA StyleÖzçakır, G. (2025). A Computational Study to Determine Thermodynamic Properties for Hydrogen Production from Sodium Borohydride Reaction. Chemistry Proceedings, 18(1), 39. https://doi.org/10.3390/ecsoc-29-26733






