Comparative Analysis of Structure, Synthesis, and Properties of Polyaniline and Polypyrrole: Insights into Conductive Polymer Variability †
Abstract
:1. Introduction
2. Methods
2.1. Materials Characterization
2.2. PANI Nanoparticles Synthesis
2.3. PPy Nanoparticles Synthesis
3. Results and Discussion
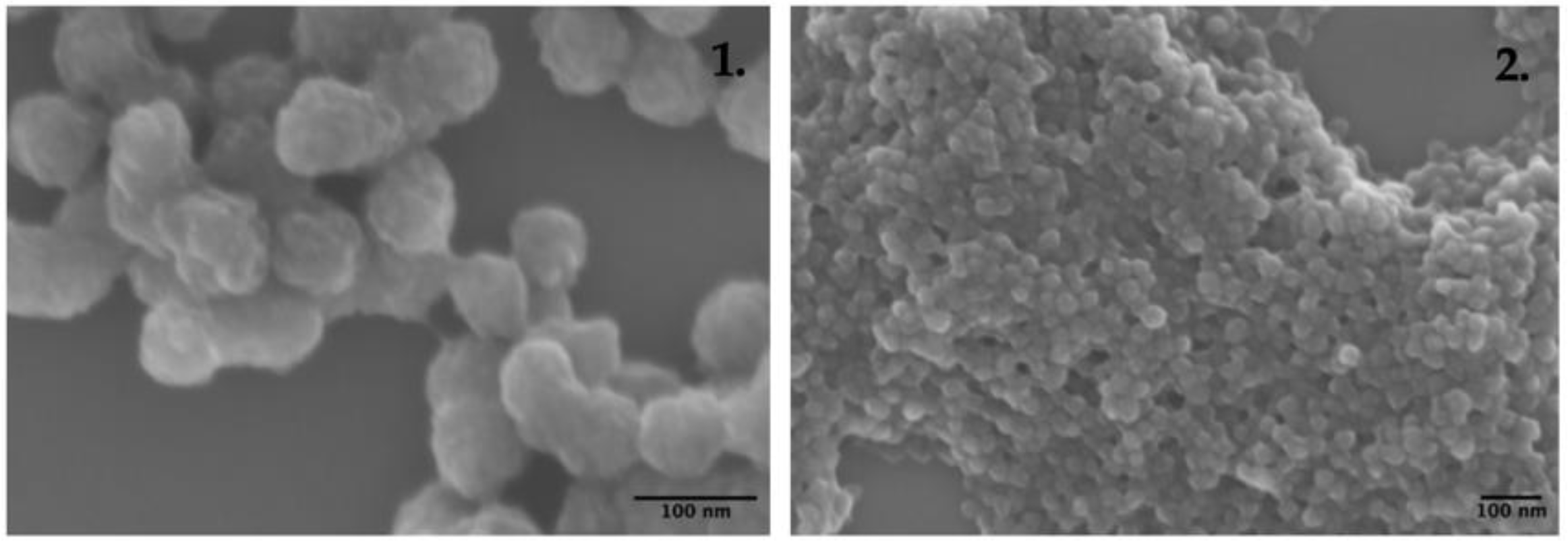
3.1. SEM Analysis
3.2. UV-Vis Analysis
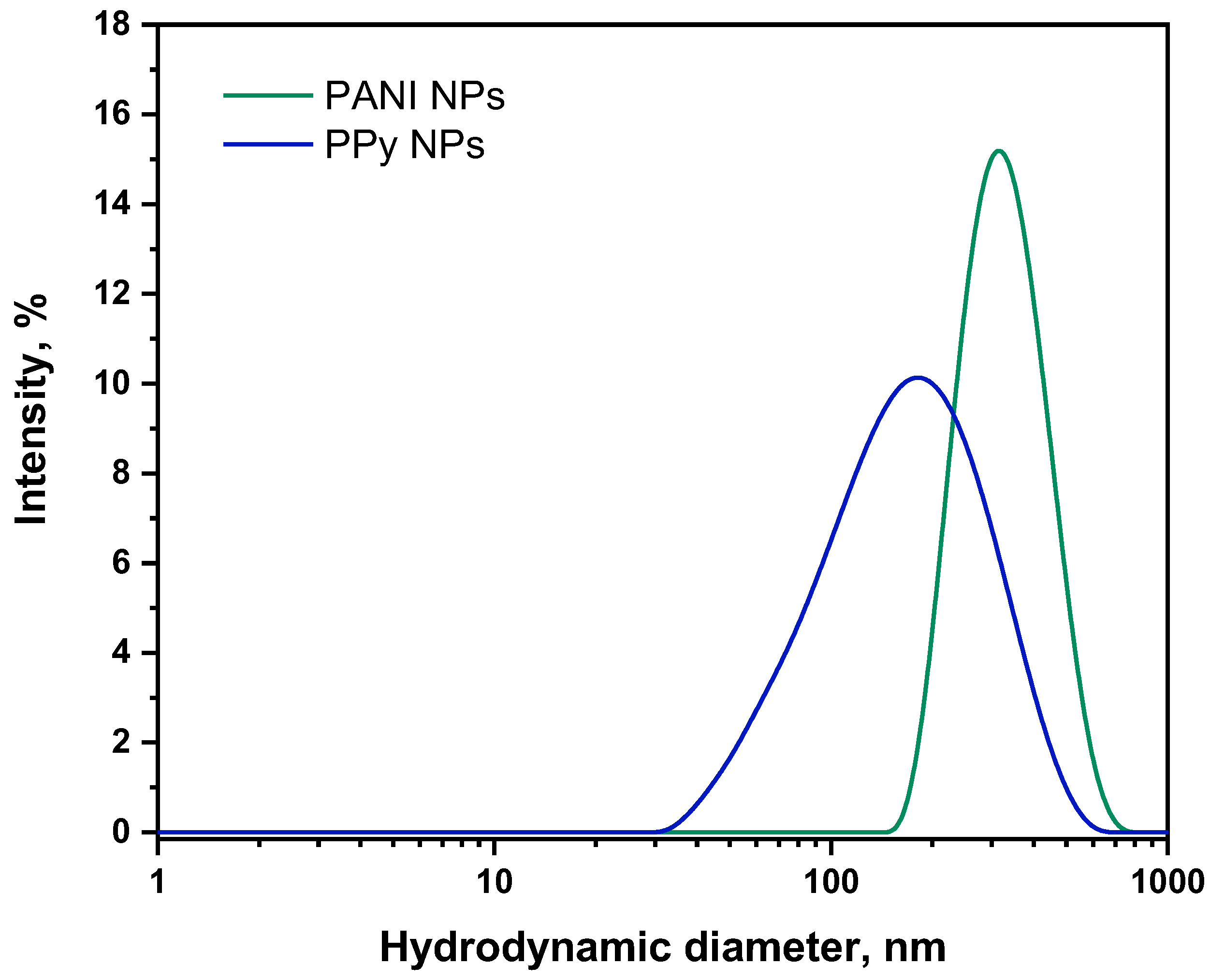
3.3. DLS Analysis
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ngwabebhoh, F.A.; Sáha, T.; Stejskal, J.; Trchová, M.; Kopecký, D.; Pfleger, J. Conducting polypyrrole-coated leathers. Prog. Org. Coatings 2023, 179, 107495. [Google Scholar] [CrossRef]
- Folorunso, O.; Olukanmi, P.; Thokozani, S. Conductive polymers’ electronic structure modification for multifunctional applications. Mater. Today Commun. 2023, 35, 106308. [Google Scholar] [CrossRef]
- Aboutorab, S.; Izadan, H.; Tavanai, H.; Nezhad, A.Z.; Bayat, M. An investigation on the effect of acid dyes as dopants on the electrical conductivity and heat generation of polypyrrole coated polyester fabric. Prog. Org. Coatings 2023, 185, 107917. [Google Scholar] [CrossRef]
- Heck, J.; Goding, J.; Lara, R.P.; Green, R. The influence of physicochemical properties on the processibility of conducting polymers: A bioelectronics perspective. Acta Biomater. 2021, 139, 259–279. [Google Scholar] [CrossRef] [PubMed]
- Leal, A.N.; de Aguiar, M.F.; Brandão, W.Q.; da Rocha, H.D.; Filho, I.J.d.C.; de Melo, C.P. Use of hybrid polyacrylonitrile/polypyrrole/polyaniline nonwoven mats for removal of the Remazol Black B dye. Mater. Chem. Phys. 2023, 310, 128447. [Google Scholar] [CrossRef]
- Uzieliene, I.; Popov, A.; Vaiciuleviciute, R.; Kirdaite, G.; Bernotiene, E.; Ramanaviciene, A. Polypyrrole-based structures for activation of cellular functions under electrical stimulation. Bioelectrochemistry 2023, 155, 108585. [Google Scholar] [CrossRef] [PubMed]
- Elumalai, P.; Charles, J. Investigation of structural and optical properties of ternary polyaniline–polypyrrole–nickel oxide (PANI-PPy-NiO) nanocomposite for optoelectronic devices. Polym. Int. 2023, 72, 176–188. [Google Scholar] [CrossRef]
- Cho, M.S.; Park, S.Y.; Hwang, J.Y.; Choi, H.J. Synthesis and electrical properties of polymer composites with polyaniline nanoparticles. Mater. Sci. Eng. C 2004, 24, 15–18. [Google Scholar] [CrossRef]
- Passador, F.R.; de Faria, P.V.; Backes, E.H.; Montanheiro, T.L.A.; Montagna, L.S.; Pinto, S.d.S.; Pessan, L.A.; Rezende, M.C. Thermal, mechanical and electromagnetic properties of LLDPE/PANI composites. Polym. Bull. 2017, 74, 2701–2717. [Google Scholar] [CrossRef]
- Rahaman, M.; Aldalbahi, A.; Almoiqli, M.; Alzahly, S. Chemical and electrochemical synthesis of polypyrrole using carrageenan as a dopant: Polypyrrole/multi-walled carbon nanotube nanocomposites. Polymers 2018, 10, 632. [Google Scholar] [CrossRef] [PubMed]
- Kausaite-Minkstimiene, A.; Mazeiko, V.; Ramanaviciene, A.; Ramanavicius, A. Evaluation of chemical synthesis of polypyrrole particles. Colloids Surf. A Physicochem. Eng. Asp. 2015, 483, 224–231. [Google Scholar] [CrossRef]
- Takahashi, K.; Kramar, J.A.; Farkas, N.; Takahata, K.; Misumi, I.; Sugawara, K.; Gonda, S.; Ehara, K. Interlaboratory comparison of nanoparticle size measurements between NMIJ and NIST using two different types of dynamic light scattering instruments. Metrologia 2019, 56, 055002. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lisyte, V.; Popov, A. Comparative Analysis of Structure, Synthesis, and Properties of Polyaniline and Polypyrrole: Insights into Conductive Polymer Variability. Chem. Proc. 2023, 14, 9. https://doi.org/10.3390/ecsoc-27-16058
Lisyte V, Popov A. Comparative Analysis of Structure, Synthesis, and Properties of Polyaniline and Polypyrrole: Insights into Conductive Polymer Variability. Chemistry Proceedings. 2023; 14(1):9. https://doi.org/10.3390/ecsoc-27-16058
Chicago/Turabian StyleLisyte, Viktorija, and Anton Popov. 2023. "Comparative Analysis of Structure, Synthesis, and Properties of Polyaniline and Polypyrrole: Insights into Conductive Polymer Variability" Chemistry Proceedings 14, no. 1: 9. https://doi.org/10.3390/ecsoc-27-16058
APA StyleLisyte, V., & Popov, A. (2023). Comparative Analysis of Structure, Synthesis, and Properties of Polyaniline and Polypyrrole: Insights into Conductive Polymer Variability. Chemistry Proceedings, 14(1), 9. https://doi.org/10.3390/ecsoc-27-16058






