Synthesis of Gold Nanorods for Multifaceted Applications †
Abstract
:1. Introduction
2. Results and Discussion
2.1. SEM Analysis
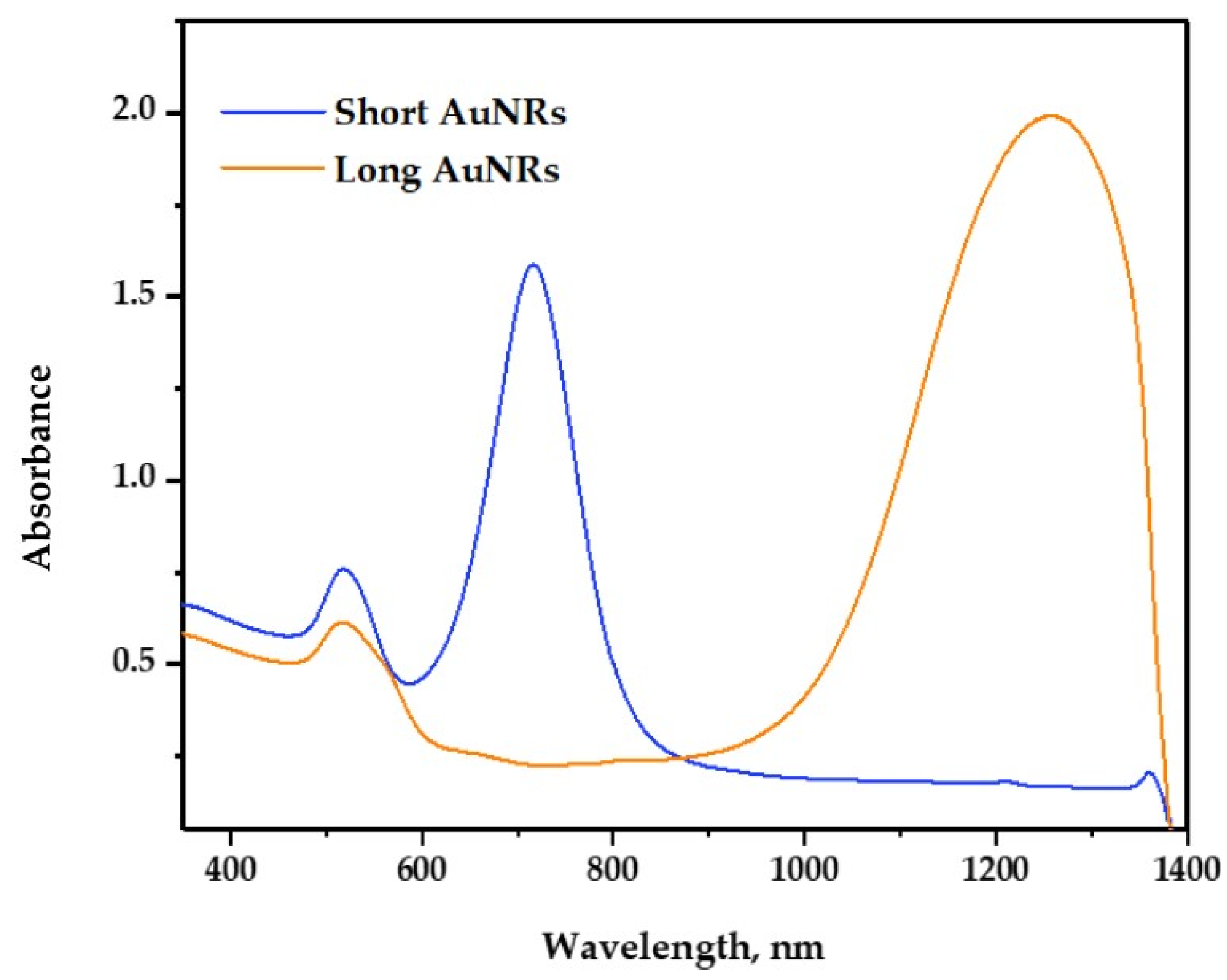
2.2. UV-Vis Analysis
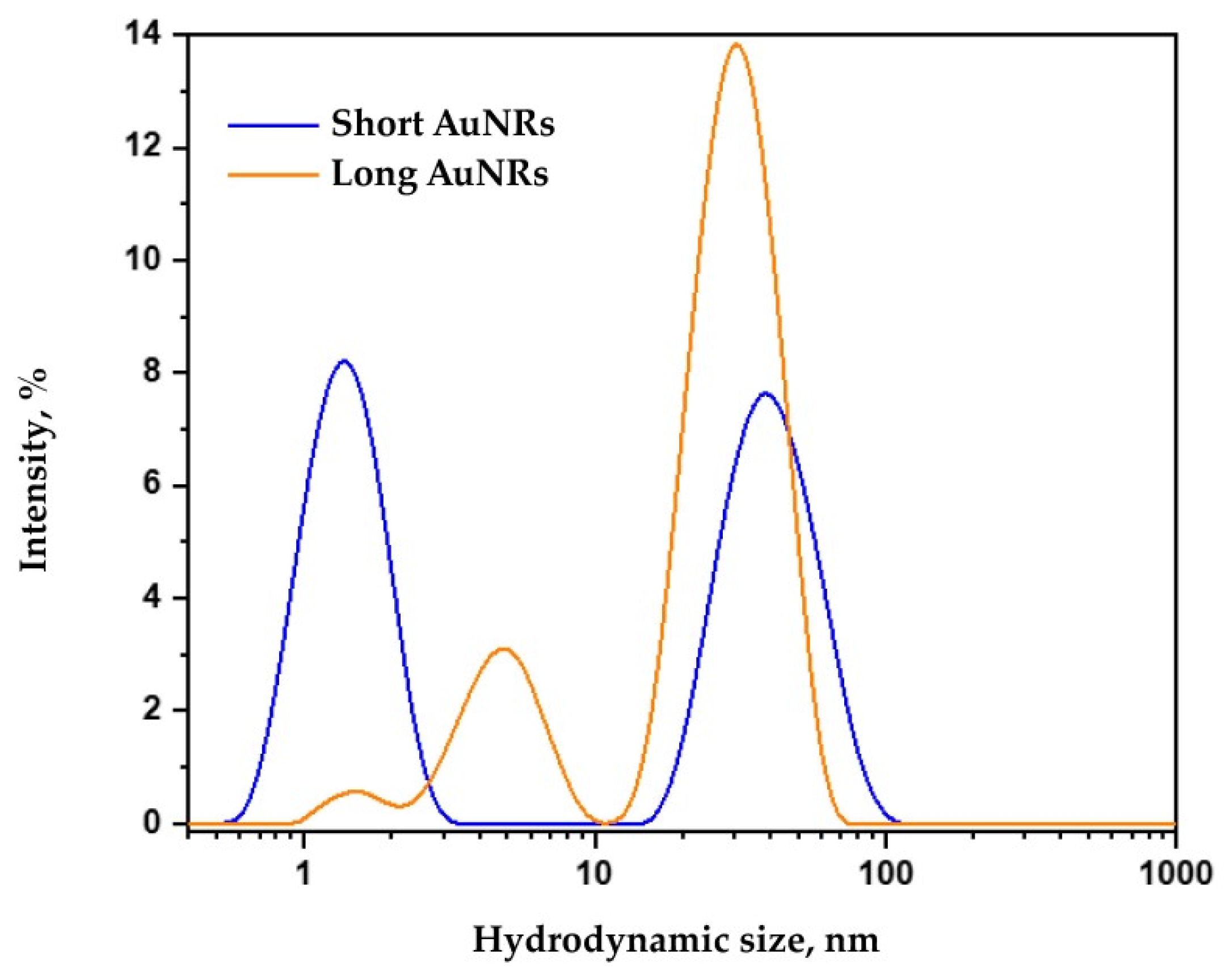
2.3. DLS Analysis
3. Materials and Methods
3.1. Seed Solution Preparation of Shorter Gold Nanorods
3.2. Synthesis of Longer Gold Nanorods
3.3. Materials Characterization
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arvizo, R.; Bhattacharya, R.; Mukherjee, P. Gold nanoparticles: Opportunities and challenges in Nanomedicine. Expert Opin Drug Deliv. 2010, 7, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Siddique, S.; Chow, J.C.L. Gold nanoparticles for drug delivery and cancer therapy. Appl. Sci. 2020, 10, 3824. [Google Scholar] [CrossRef]
- Ferrari, E. Gold nanoparticle-based Plasmonic Biosensors. Biosensors 2023, 13, 411. [Google Scholar] [CrossRef] [PubMed]
- Amendola, V.; Pilot, R.; Frasconi, M.; Maragò, O.M.; Iatì, M.A. Surface plasmon resonance in gold nanoparticles: A review. J. Phys. Condens. Matter. 2017, 29, 203002. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Sun, T.; Grattan, K.T.V. Gold nanorod-based localized surface plasmon resonance biosensors: A review. Sens. Actuators B Chem. 2014, 195, 332–351. [Google Scholar] [CrossRef]
- Wu, H.Y.; Chu, H.C.; Kuo, T.J.; Kuo, C.L.; Huang, M.H. Seed-mediated synthesis of high aspect ratio gold nanorods with nitric acid. Chem. Mater. 2005, 17, 6447–6451. [Google Scholar] [CrossRef]
- Kesharwani, P.; Ma, R.; Sang, L.; Fatima, M.; Sheikh, A.; Abourehab, M.A.S.; Gupta, N.; Chen, Z.-S.; Zhou, Y. Gold nanoparticles and gold nanorods in the landscape of cancer therapy—Molecular cancer. Mol. Cancer 2023, 98, 22. [Google Scholar]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The optical properties of metal nanoparticles: The influence of size, shape, and dielectric environment. J. Phys. Chem. 2003, B107, 668–677. [Google Scholar] [CrossRef]
- Takahashi, K.; A Kramar, J.; Farkas, N.; Takahata, K.; Misumi, I.; Sugawara, K.; Gonda, S.; Ehara, K. Interlaboratory comparison of nanoparticle size measurements between NMIJ and NIST using two different types of dynamic light scattering instruments. Metrologia 2019, 56, 055002. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sidorova, M.; Popov, A. Synthesis of Gold Nanorods for Multifaceted Applications. Chem. Proc. 2023, 14, 11. https://doi.org/10.3390/ecsoc-27-16057
Sidorova M, Popov A. Synthesis of Gold Nanorods for Multifaceted Applications. Chemistry Proceedings. 2023; 14(1):11. https://doi.org/10.3390/ecsoc-27-16057
Chicago/Turabian StyleSidorova, Marina, and Anton Popov. 2023. "Synthesis of Gold Nanorods for Multifaceted Applications" Chemistry Proceedings 14, no. 1: 11. https://doi.org/10.3390/ecsoc-27-16057
APA StyleSidorova, M., & Popov, A. (2023). Synthesis of Gold Nanorods for Multifaceted Applications. Chemistry Proceedings, 14(1), 11. https://doi.org/10.3390/ecsoc-27-16057






