Crystal Structure of 2-(Ethoxymethylene)malononitrile and DFT Evaluation of the C-H···N≡C Close Contacts Energy †
Abstract
1. Introduction
2. Materials and Methods
2.1. Physical Measurements
2.2. Synthesis, Characterization, and Crystallization
2.3. Crystal Structure Determinations and Refinement
2.4. DFT Calculations
3. Results and Discussion
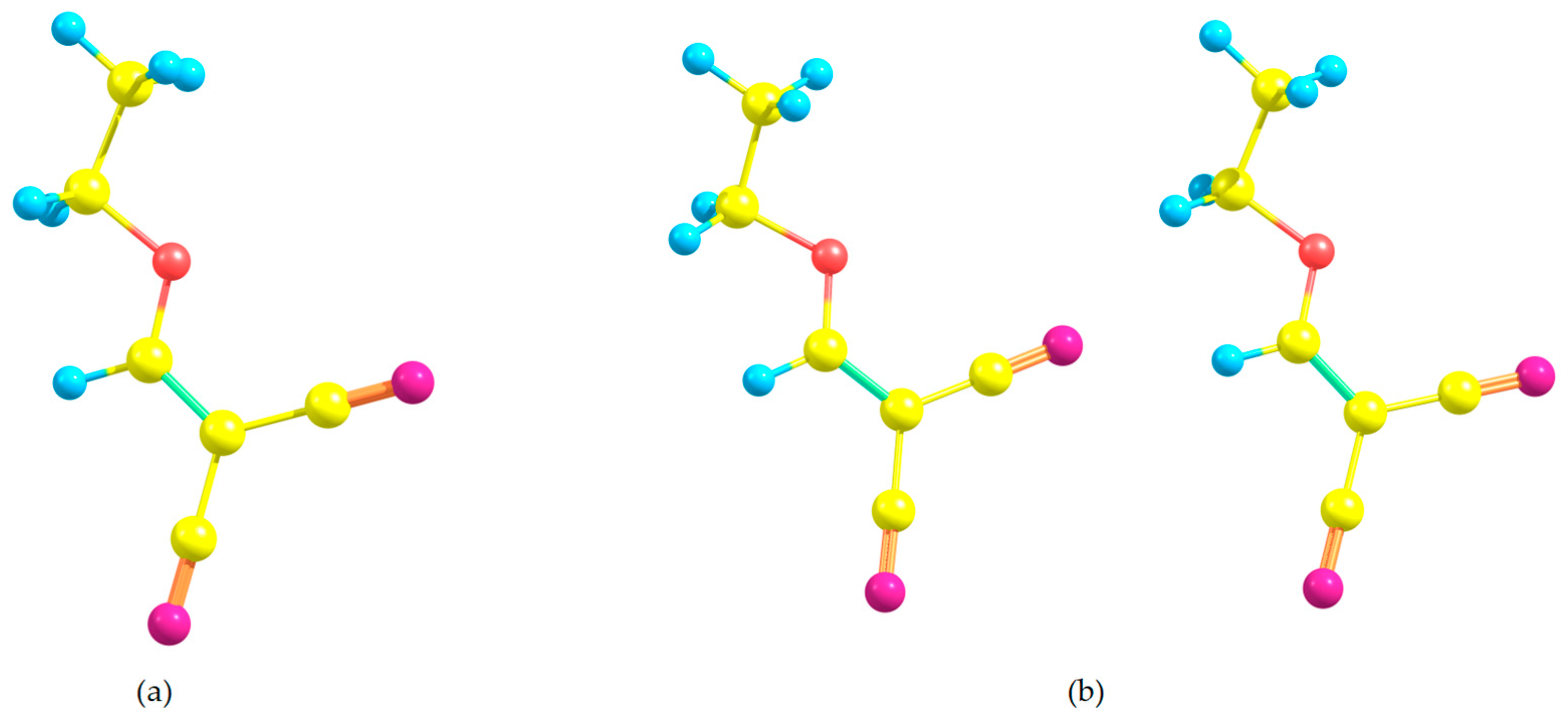
3.1. Crystal Structure Analysis
3.2. Packing Features of (1)
3.3. Hirshfeld Surface Analysis
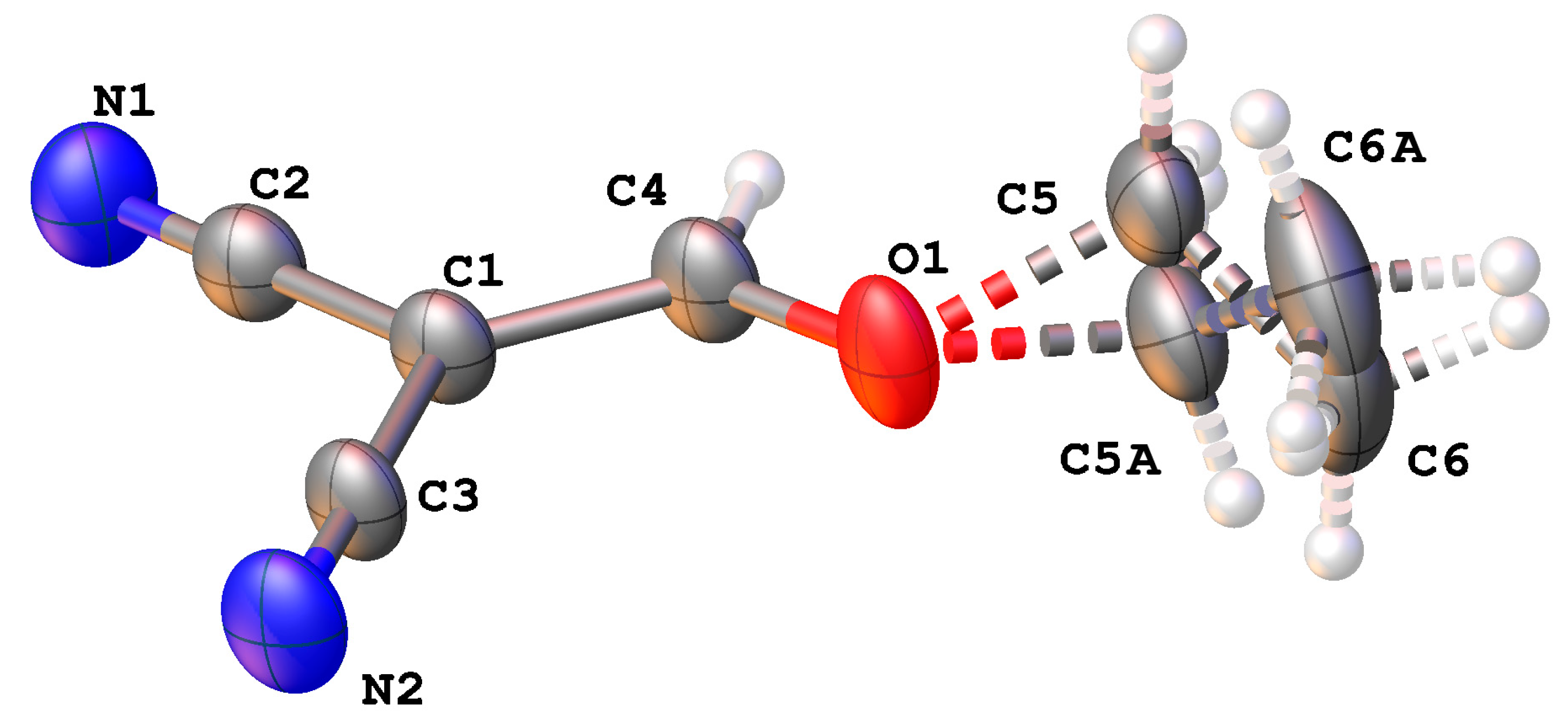
3.4. Theoretical Evaluation of the Close Contact Energy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diels, O.; Gärtner, H.; Kaack, R. Über Versuche zur Darstellung des Carbonylcyanids und eine Methode zur Gewinnung ungesättigter Amino-Säuren. Berichte Der Dtsch. Chem. Ges. (A B Ser.) 1922, 55, 3439–3448. [Google Scholar] [CrossRef][Green Version]
- Ochiai, M.; Yamamoto, S.; Suefuji, T.; Chen, D. Stereoselective synthesis of (Z)-enethiols and their derivatives: Vinylic SN2 reaction of (E)-alkenyl (phenyl)-λ3-iodanes with thioamides. Org. Lett. 2001, 3, 2753–2756. [Google Scholar] [CrossRef] [PubMed]
- Konakahara, T.; Sugama, N.; Yamada, A.; Kakehi, A.; Sakai, N. Cyclization reaction of N-silyl-1-azaallyl anions with Michael acceptors as a new synthetic method of 2,3,5,6-tetra- and 2,3,6-trisubstituted pyridines. Heterocycles 2001, 55, 313–322. [Google Scholar] [CrossRef]
- Osipov, A.K.; Anis’kov A., A.; Yegorova, A.Y. Synthesis and configuration of (arylamino) methylidene-3H-furan-2-ones. Russ. J. Org. Chem. 2017, 53, 210–214. [Google Scholar] [CrossRef]
- Al-Refai, M.; Ali, B.F.; Said, A.B.; Geyer, A.; Marsch, M.; Harms, K. Synthesis, characterization, crystal structure and supramolecularity of ethyl (E)-2-cyano-3-(3-methylthiophen-2-yl) acrylate and a new polymorph of ethyl (E)-2-cyano-3-(thiophen-2-yl) acrylate. Acta Crystallogr. Sect. E Crystallogr. Commun. 2019, 75, 1357–1361. [Google Scholar] [CrossRef] [PubMed]
- Kalkhambkar, R.G.; Gayathri, D.; Gupta, V.K.; Kant, R.; Jeong, Y.T. (E)-Ethyl 2-cyano-3-(furan-2-yl) acrylate. Acta Crystallogr. Sect. E: Struct. Rep. Online 2012, 68, 1482. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.; He, Y.; Xu, J.; Liu, H.; Wang, X.; Feng, M.; Qi, C.; Zhang, J.; Peng, C. Preparation and bioevaluation of 99mTc nitrido radiopharmaceuticals with pyrazolo [1,5-a]pyrimidine as tumor imaging agents. Med. Chem. Res. 2012, 21, 523–530. [Google Scholar] [CrossRef]
- CrysAlisPro, Agilent Technologies, Version 1.171.37.33 (Release 27-03-2014 CrysAlis171.NET). Available online: https://www.scirp.org/reference/referencespapers?referenceid=1770037 (accessed on 13 September 2023).
- Bourhis, L.J.; Dolomanov, O.V.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H.J. The anatomy of a comprehensive constrained, restrained refinement program for the modern computing environment—Olex2 dissected. Appl. Crystallogr. 2015, A71, 59–75. [Google Scholar] [CrossRef]
- Scheldrick, G.M. SHELXT-Integrated space-group and crystals-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H.J. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339. [Google Scholar] [CrossRef]
- Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.; et al. Gaussian 09, Rev. C.01; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1998, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. Hybrid Meta Density Functional Theory Methods for Thermochemistry, Thermochemical Kinetics, and Noncovalent Interactions: The MPW1B95 and MPWB1K Models and Comparative Assessments for Hydrogen Bonding and van der Waals Interactions. J. Phys. Chem. 2004, 108, 6908–6918. [Google Scholar] [CrossRef]
- Remya, K.; Suresh, C.H. Which density functional is close to CCSD accuracy to describe geometry and interaction energy of small noncovalent dimers? A benchmark study using Gaussian09. J. Comput. Chem. 2013, 34, 1341–1353. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Castro Agudelo, B.; Cárdenas, J.C.; Macías, M.A.; Ochoa-Puentes, C.; Sierra, C.A. Crystal structure of ethyl (E)-2-cyano-3-(thiophen-2-yl) acrylate: Two conformers forming a discrete disorder. Acta Crystallogr. Sect. E Crystallogr. Commun. 2017, 73, 1287–1289. [Google Scholar] [CrossRef] [PubMed]
- Hirshfeld, H.L. Bonded-atom fragments for describing molecular charge denseties. Theor. Chim. Acta 1977, 44, 129–138. [Google Scholar] [CrossRef]
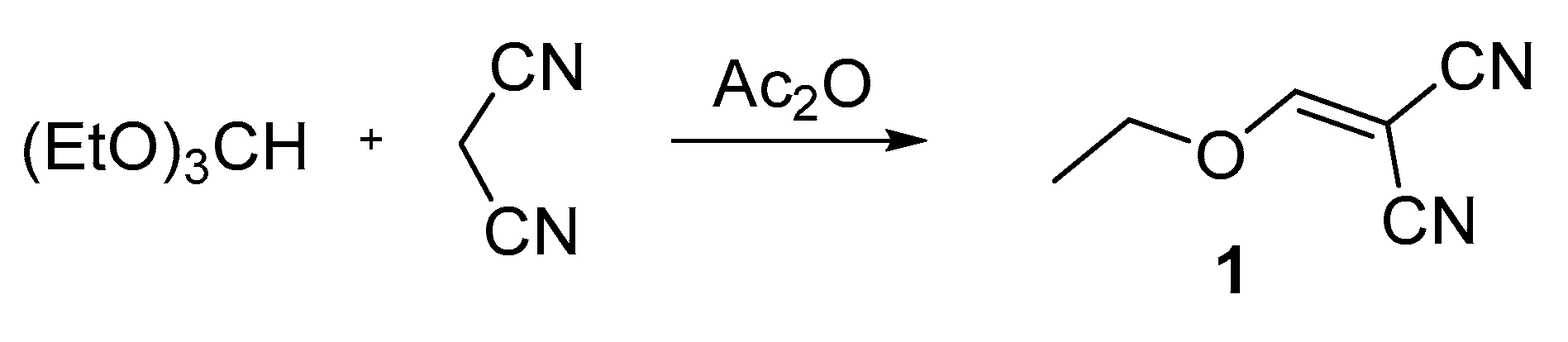

| Moiety | H···O, Å | D···A, Å | D—H···A, ° |
|---|---|---|---|
| CH2 | 3.294 | 3.424 | 126.5 |
| CH3 | 2.761 | 3.949 | 127.0 |
| Method | H···N, Å | D···A, Å | D—H···A, ° | E (kcal/mol) |
|---|---|---|---|---|
| XRD | 2.494 | 3.423 | 176.22 | - |
| M06-2X | 2.424 | 3.472 | 176.23 | −1.20 |
| MPWB95 | 2.561 | 3.611 | 160.52 | −0.36 |
| B97-D3 | 2.423 | 3.483 | 163.65 | −1.27 |
| WB97XD | 2.385 | 3.439 | 162.61 | −1.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grinev, V.S.; Demeshko, I.A.; Sklyar, A.E.; Yegorova, A.Y. Crystal Structure of 2-(Ethoxymethylene)malononitrile and DFT Evaluation of the C-H···N≡C Close Contacts Energy. Chem. Proc. 2023, 14, 10. https://doi.org/10.3390/ecsoc-27-16052
Grinev VS, Demeshko IA, Sklyar AE, Yegorova AY. Crystal Structure of 2-(Ethoxymethylene)malononitrile and DFT Evaluation of the C-H···N≡C Close Contacts Energy. Chemistry Proceedings. 2023; 14(1):10. https://doi.org/10.3390/ecsoc-27-16052
Chicago/Turabian StyleGrinev, Vyacheslav S., Ilya A. Demeshko, Anna E. Sklyar, and Alevtina Yu. Yegorova. 2023. "Crystal Structure of 2-(Ethoxymethylene)malononitrile and DFT Evaluation of the C-H···N≡C Close Contacts Energy" Chemistry Proceedings 14, no. 1: 10. https://doi.org/10.3390/ecsoc-27-16052
APA StyleGrinev, V. S., Demeshko, I. A., Sklyar, A. E., & Yegorova, A. Y. (2023). Crystal Structure of 2-(Ethoxymethylene)malononitrile and DFT Evaluation of the C-H···N≡C Close Contacts Energy. Chemistry Proceedings, 14(1), 10. https://doi.org/10.3390/ecsoc-27-16052






