Phenothiazine Conjugate with Mitochondria-Directed Cationic Compound F16: Synthesis and Cytotoxic Action against Human Breast Carcinoma †
Abstract
:1. Introduction
2. Materials and Methods
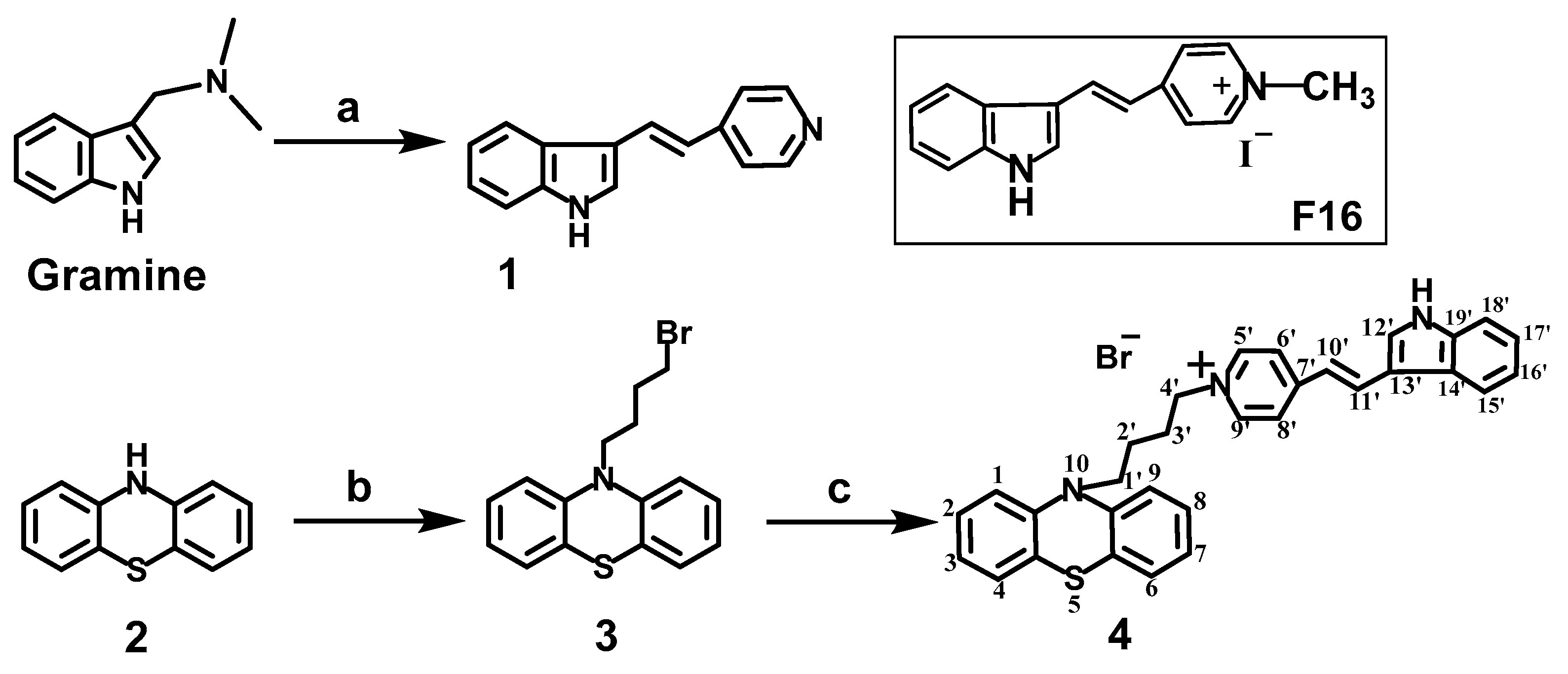
2.1. Chemistry
2.1.1. Synthesis of N-(4-Bromobutyl) Phenothiazine 3
2.1.2. Synthesis of 4-(10H-Phenothiazin-10-yl)butyl-N-(E)-4-(1H-indol-3-yl-vinyl)pyridinium Bromide 4
2.2. Biology
2.2.1. Cell Line BT-474 and Culture Condition
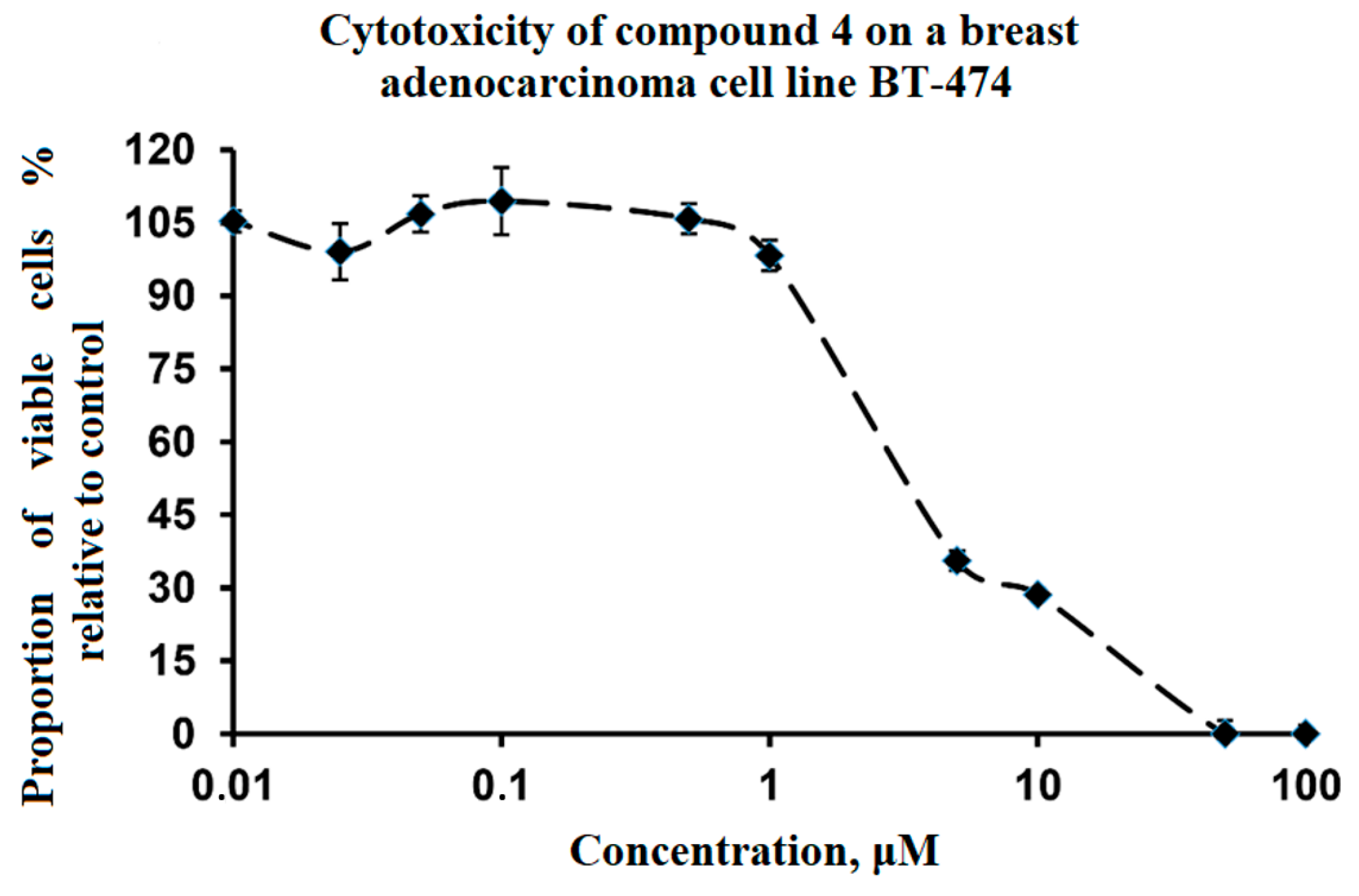
2.2.2. Effect of Compound 4 on the Viability of BT-474 Human Breast Adenocarcinoma Tumor Cells
2.2.3. Effect of Sequential Addition of Compound 4 on the Membrane Potential of Rat Liver Mitochondria
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shalini; Kumar, V. Have molecular hybrids delivered effective anticancer treatments and what should future drug discovery focus on? Expert Opin. Drug Discov. 2021, 16, 335–363. [Google Scholar] [CrossRef] [PubMed]
- Meunier, B. Hybrid Molecules with a Dual Mode of Action: Dream or Reality? Acc. Chem. Res. 2008, 41, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Ohlow, M.J.; Moosmann, B. Phenothiazine: The seven lives of pharmacology’s first lead structure. Drug Discov. Today 2011, 16, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Posso, M.C.; Domingues, F.C.; Ferreira, S.; Silvestre, S. Development of Phenothiazine Hybrids with Potential Medicinal Interest: A Review. Molecules 2022, 27, 276–308. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-H.; Bai, L.-Y.; Tsai, M.-H.; Chu, P.-C.; Chiu, C.-F.; Chen, M.Y.; Chiu, S.-J.; Chian, J.-H.; Weng, J.-R. Pharmacological exploitation of the phenothiazine antipsychotics to develop novel antitumor agents–A drug repurposing strategy. Sci. Rep. 2016, 6, 27540. [Google Scholar] [CrossRef] [PubMed]
- Spengler, G.; Csonka, Á.; Molnar, J.; Amaral, L. The Anticancer Activity of the Old Neuroleptic Phenothiazine-type Drug Thioridazine. Anticancer Res. 2016, 36, 5701–5706. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, A.; Pan, L.; Groen, R.W.J.; Baleydier, F.; Kentsis, A.; Marineau, J.; Grebliunaite, R.; Kozakewich, E.; Reed, C.; Pflumio, F.; et al. Phenothiazines induce PP2A-mediated apoptosis in T cell acute lymphoblastic leukemia. J. Clin. Investig. 2014, 124, 644–655. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.; Hartley, R.C.; Cochemé, H.M.; Murphy, M.P. Mitochondrial pharmacology. Trends Pharmacol. Sci. 2012, 33, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Zielonka, J.; Sikora, A.; Hardy, M.; Ouari, O.; Vasquez-Vivar, J.; Cheng, G.; Lopez, M.; Kalyanaraman, B. Mitochondria-Targeted Triphenylphosphonium-Based Compounds: Syntheses, Mechanisms of Action, and Therapeutic and Diagnostic Applications. Chem. Rev. 2017, 117, 10043–10120. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.B.; Zhao, Z.L.; Liu, T.; Xie, G.J.; Jin, C.; Deng, T.G.; Sun, Y.; Li, X.; Hu, X.X.; Zhang, X.B.; et al. A Multi-Mitochondrial Anticancer Agent that Selectively Kills Cancer Cells and Overcomes Drug Resistance. ChemMedChem 2017, 12, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Spivak, A.Y.; Nedopekina, D.A.; Gubaidullin, R.R.; Davletshin, E.V.; Tukhbatullin, A.A.; D’yakonov, V.A.; Yunusbaeva, M.M.; Dzhemileva, L.U.; Dzhemilev, U.M. Pentacyclic triterpene acid conjugated with mitochondria-targeting cation F16: Synthesis and evaluation of cytotoxic activities. Med. Chem. Res. 2021, 30, 940–951. [Google Scholar] [CrossRef]
- Heise, N.V.; Major, D.; Hoenke, S.; Kozubek, M.; Serbian, I.; Csuk, R. Rhodamine 101 Conjugates of Triterpenoic Amides Are of Comparable Cytotoxicity as Their Rhodamine B Analogs. Molecules 2022, 27, 2220. [Google Scholar] [CrossRef] [PubMed]
- Dubinin, M.V.; Semenova, A.A.; Ilzorkina, A.I.; Penkov, N.V.; Nedopekina, D.A.; Sharapov, V.A.; Khoroshavina, E.I.; Davletshin, E.V.; Belosludtseva, N.V.; Spivak, A.Y.; et al. Mitochondria-targeted prooxidant effects of betulinic acid conjugated with delocalized lipophilic cation F16. Free Radic. Biol. Med. 2021, 168, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Wang, R.; Sun, S.; Wang, G.; Hu, C. Preparation Method and Application of Thiazine Fluorescent Derivative. Patent CN110804049A, 2020. Espacenet. Available online: https://worldwide.espacenet.com/patent/search/family/069490566/publication/CN110804049A?q=CN110804049A (accessed on 18 February 2020).
- Dunn, E.A.; Roxburgh, M.; Larsen, L.; Smith, R.A.J.; McLellan, A.D.; Heikal, A.; Murphy, M.P.; Cook, G.M. Incorporation of triphenylphosphonium functionality improves the inhibitory properties of phenothiazine derivatives in Mycobacterium tuberculosis. Bioorg. Med. Chem. 2014, 22, 5320–5328. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Li, D.-W.; Yang, L.-Y.; Fu, L.; Zhu, X.-J.; Wong, W.-K.; Jiang, F.-L.; Liu, Y. A novel bifunctional mitochondriatargeted anticancer agent with high selectivity for cancer cells. Sci. Rep. 2015, 5, 13543. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Zhao, L.; Zeng, X. A Highly Selective Turn-on Fluorescent Chemodosimeter for Cu2+ Through a Cu2+-Promoted Redox Reaction. J. Fluoresc. 2014, 24, 1671–1677. [Google Scholar] [CrossRef] [PubMed]
- Fantin, V.R.; Berardi, M.J.; Scorrano, L.; Korsmeyer, S.J.; Leder, P. A novel mitochondriotoxic small molecule that selectively inhibits tumor cell growth. Cancer Cell 2002, 2, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Fantin, V.R.; Leder, P. F16, a mitochondriotoxic compound, triggers apoptosis or necrosis depending on the genetic background of the target carcinoma cell. Cancer Res. 2004, 64, 329–336. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davletshin, E.; Nedopekina, D.; Khalitova, R.; Dubinin, M.; Belosludtsev, K.; Spivak, A. Phenothiazine Conjugate with Mitochondria-Directed Cationic Compound F16: Synthesis and Cytotoxic Action against Human Breast Carcinoma. Chem. Proc. 2023, 14, 49. https://doi.org/10.3390/ecsoc-27-16120
Davletshin E, Nedopekina D, Khalitova R, Dubinin M, Belosludtsev K, Spivak A. Phenothiazine Conjugate with Mitochondria-Directed Cationic Compound F16: Synthesis and Cytotoxic Action against Human Breast Carcinoma. Chemistry Proceedings. 2023; 14(1):49. https://doi.org/10.3390/ecsoc-27-16120
Chicago/Turabian StyleDavletshin, Eldar, Darya Nedopekina, Rezeda Khalitova, Mikhail Dubinin, Konstantin Belosludtsev, and Anna Spivak. 2023. "Phenothiazine Conjugate with Mitochondria-Directed Cationic Compound F16: Synthesis and Cytotoxic Action against Human Breast Carcinoma" Chemistry Proceedings 14, no. 1: 49. https://doi.org/10.3390/ecsoc-27-16120
APA StyleDavletshin, E., Nedopekina, D., Khalitova, R., Dubinin, M., Belosludtsev, K., & Spivak, A. (2023). Phenothiazine Conjugate with Mitochondria-Directed Cationic Compound F16: Synthesis and Cytotoxic Action against Human Breast Carcinoma. Chemistry Proceedings, 14(1), 49. https://doi.org/10.3390/ecsoc-27-16120








