1. Introduction
Preparations based on peptides are becoming more and more in demand due to their specificity, which, first of all, lies in their functional features. Peptides, when taken into the human body, perform the following pharmacological functions: eliminate inflammatory processes, strengthen tissues and bones, restore the process of metabolism, and have an immunomodulatory effect on the whole organism. Functional features of regulatory peptides can be very different and are determined by the functions of organs and tissues from which they are isolated.
For example, a peptide preparation such as “Bonomarlot” is used as a cancer prevention agent and promotes the formation of bone system cells; it is isolated from the bone marrow of cattle. Also from the organs of cattle, namely from the parathyroid gland, it is possible to isolate peptides designed to normalize the work of parathyroid gland cells. Such peptides are contained in the preparation “Bonortic”, which is prescribed for the prevention of osteoporosis. The drug “Visoluten” can also refer to a group of drugs derived from the biological tissue of animals. A peptide complex isolated from eye tissues of young healthy animals strengthens or partially restores cells of the retina, conjunctiva, and visual analyzer. The number of preparations whose active substance are regulatory peptides isolated from biological material is large, and it is not possible to cover them all [
1].
Due to the fact that the raw materials for the isolation of peptide preparations are expensive resources, we chose the skin secretion of the African catfish (Clarias gariepinus) as the biological material. This substance is interesting because the mucus of this scaleless fish species is not only a relatively inexpensive source of peptides, but it is also the only defense of the African catfish against harmful microorganisms and mechanical damage.
One of the main advantages of regulatory peptides is the presence of some antioxidant activity, which is manifested to varying degrees in peptide complexes at different concentrations.
In this paper we will highlight the methods of determining the antioxidant activity that regulatory peptides isolated from the skin secretion of the African catfish (Clarias gariepinus) are likely to possess.
2. Materials and Methods
Obtaining the peptide fraction used as an inhibitor. The regulatory peptide fraction was isolated by the acetic acid extraction of Clarias gariepinus epidermal mucus. A 3% acetic acid solution containing 0.1 to 0.3% inorganic salt was used. The inorganic salt used was zinc, calcium, and magnesium chlorides. The extraction was carried out in the mode of periodic ultrasonic cavitation treatment on an IL100-6/1 disperser for 48 h at a temperature of 4 °C. The obtained extract was separated by centrifugation for 10 min at 7000 rpm and further purified by adsorption with activated carbon for 24 h under constant stirring. After the removal of the adsorbent from the clarified solution, the peptide fraction was isolated by re-precipitation with a 2-fold excess of acetone. The resulting precipitate of the peptide fraction was separated using a Schott filter (16 µm pore width).
The use of UV spectroscopy to analyze antioxidant properties. To determine the antioxidant activity of the samples, we used the technique of studying the autoxidation of adrenaline in vitro. The purpose of the method is to compare the autoxidation rate of a pharmaceutical-grade 1% adrenaline solution in the presence (analyzed solution) and in the absence (control solution) of an aqueous solution of peptide fraction in sodium carbonate buffer (pH = 10.65) [
2].
To determine the antiradical activity of peptide complexes, 2,2-diphenyl-1-picrylhydrazyl (DPPH) dissolved in ethanol was used. The optical density of the initial DPPH solution was compared with the optical density after the reaction of DPPH with the antioxidant under study. The decrease in optical density values at a wavelength of 515 nm indicates the presence of antioxidant activity of the studied compound.
The following reagents were used: 1% adrenaline solution; salts—ZnCl2, MgCl2, and CaCl2; DPPH; L-glutathione reduced; and C2H5OH (Sigma Aldrich, Saint Louis, MO, USA). The peptide fraction was prepared as described above. A sodium carbonate buffer was prepared from Na2CO3, and a fixed pH value was achieved by adding dry NaHCO3 to the solution to the desired pH = 10.65. All solutions were prepared with bidistilled water.
3. Results and Discussions
The assumption about the antioxidant properties of the isolated peptide complexes was made based on the functional features of the epidermal secretion of the African catfish. Therefore, it was of interest to quantify the antioxidant activity of the investigated peptides. For this purpose, two independent spectroscopic methods were used [
3].
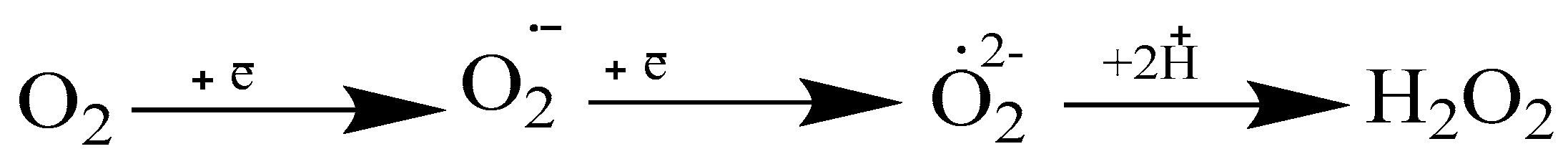
The first one consisted of the inhibition of the adrenaline autooxidation reaction via the peptide complex. To determine the antioxidant activity of the obtained peptides, the concentration of adrenaline oxidation products was determined by the optical density readings of the solutions. According to its properties and chemical structure, adrenaline is an electron donor, and this function is realized in the process of quinoid oxidation. The autoxidation of adrenaline into adrenochrome occurs in the process of dehydrogenation and cyclization with the formation of intermediate products, including adrenaline–semiquinone, adrenalinequinone, leucoadrenochrome, and adrenochrome–semiquinone. This process is accompanied by the one-electron reduction of oxygen and is associated with the further formation of superoxide radicals (O
2−.), which in turn oxidize and destroy nucleic acids, proteins, polysaccharides, and biomembranes (
Figure 1).
In this work, the ability of zinc, calcium, and magnesium chlorides to regulate the deposition of major proteins of the epidermal secretion of the Clarias gariepinus epidermal secretion was compared. Controlled sedimentation allows for the changing of the total peptide composition of the fractions. This directly affects the most important properties of the obtained peptide complexes [
4].
To quantify the inhibitory effect of the peptide fraction, we determined the ratio of the rate constants of the autoxidation reaction of adrenadine without an inhibitor and in the presence of the peptide complex:
where W(inhibition)—fraction of inhibition of adrenaline autoxidation;
k0—rate constant of adrenaline autoxidation without peptides;
kp—adrenaline autoxidation rate constant in the presence of peptides.
The calculation of the inhibitory effect of peptide complexes is presented in
Table 1.
Thus, the inhibitory effect of peptides precipitated by biogenic metal salts is many times higher or comparable to the similar effect of the pharmacopeial drug Timalin. The presented data allow us to assume the presence of sulfhydryl and/or phenolic functional groups, which are responsible for the antioxidant properties of regulatory peptides [
5].
The second method for the determination of antioxidant activity is based on the spectrophotometric determination of the optical density of the reaction product of isolated regulatory peptides with 2,2-diphenyl-1-picrylhydrazyl (DPPH) in an organic solvent (ethanol). L-glutathione reduced was used as a reference antioxidant. We used it as a reference for the comparison of antioxidant properties. The quantitative measure of antiradical activity in this method was used as a value of decrease in optical density of 0.004% The DPPH solution after reaction with antioxidant is represented as ∆D. A comparison of antioxidant properties of glutathione and peptide complexes isolated by different salts is presented in
Table 2.
The results obtained confirm the antioxidant activity of peptide complexes consisting of the direct binding of free radicals. Obviously, such activity of peptides does not depend on the choice of salt used for their isolation. Although it markedly exceeds the analogous activity of glutathione. The difference in the results obtained by the two methods can be explained by the multicomponent nature of the peptide fractions. Probably, the concentration of individual peptides capable of reacting with superoxydradical (in adrenaline autoxidation) is higher in the case of using ZnCl
2 for isolation. This having been said, the amount of peptides capable of interaction with DPPH is approximately the same in all fractions, regardless of the nature of the salt used for precipitation [
6].
4. Conclusions
The nature of the salt used for the precipitation of major proteins from the epidermal mucus of the African catfish plays a determining role in the formation of the properties of the peptide preparation. It is shown that among the biogenic metal chlorides chosen for the study, it is ZnCl2 that allows for the isolation of the peptide fraction with the highest antioxidant activity. This activity is almost four times higher than the activity of the preparation “Timalin” and two times higher than the activity of the analogous fraction isolated using CaCl2 and MgCl2. At the same time, the ability of the studied peptide fractions to inhibit the process of adrenaline autoxidation is manifested when they are added to the substrate in concentrations of 5 Mg/mL
A comparison of the obtained results on the determination of antioxidant activity using the mentioned methods showed that the highest value of antioxidant activity is observed in the range of low concentrations of antioxidants, i.e., obtained peptide complexes. Thus we can state that the isolated complexes of regulatory peptides have antioxidant activity, hence they can prevent the oxidation of nucleic acids, proteins, polysaccharides, and biomembranes via superoxide radicals [
7]. This, in turn, makes them promising drugs for the treatment of severe forms of wound processes.
Author Contributions
Conceptualization, L.P. and E.G.; methodology, L.P. and E.G.; software, L.P. and E.G.; validation, L.P. and E.G.; formal analysis, L.P. and E.G.; investigation, L.P. and E.G.; resources, L.P. and E.G.; data curation, L.P. and E.G.; writing—original draft preparation, L.P. and E.G.; writing—review and editing, L.P. and E.G.; visualization, L.P. and E.G.; supervision, E.G.; project administration, L.P. and E.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Khavinson, V.H. Drug peptide preparations: Past, present, future. Clin. Med. 2020, 98, 165–177. (In Russian) [Google Scholar] [CrossRef]
- Prida, A.I.; Ivanova, R.I. Natural Antioxidants of Polyphenolic Nature (Antiradical Properties and Prospects for Use). Food Ingred. Raw Mater. Addit. 2004, 2, 76–78. (In Russian) [Google Scholar]
- Kudryavtseva, T.A. MPC G01N 33/50(2006.01). Determination of Antioxidant Activity of Some Substances of Amino Acid, Peptide and Polyphenolic Nature In-Vitro: No. 2018113686. Russian Patent 2680604, 25 February 2019. [Google Scholar]
- Liu, Y.; Zhang, Z.; Li, T.; Xu, H.; Zhang, H. Senescence in osteoarthritis: From mechanism to potential treatment. Arthritis Res. Ther. 2022, 24, 174. [Google Scholar] [CrossRef] [PubMed]
- Grekhneva, E.V.; Perkova, L.A. Comparative Characterization of Antioxidant Activity of Peptide Complex Isolated from the Epidermal Secretion of the African Catfish by Different Methods. Izvestiya Vysshee Obrazovaniya Vysshee Obrazovaniya. Ser. “Chem. Chem. Technol.” 2023, 8, 46–53. (In Russian) [Google Scholar]
- Sirota, T.V. Method for Determining the Antioxidant Activity of Superoxide Dismutase and Chemical Compounds. Russian Patent 2144674, 20 January 2000. [Google Scholar]
- Smakhtin, M.Y.; Chulanova, A.A.; Smakhtina, A.M.; Shumakova, A.V. Modern Regulatory Peptides: Their Reparative Effects and Influence on the Activity of Free-Radical Reactions. Mod. Med. New Approaches Curr. Res. 2020, 1, 581–584. (In Russian) [Google Scholar]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).