Synthesis and Cytotoxic Activity of Conjugates of Mitochondrial-Directed Cationic Compound F16 with Ursane-Structure Triterpenic Acids Containing a Polyhydroxylated A-ring †
Abstract
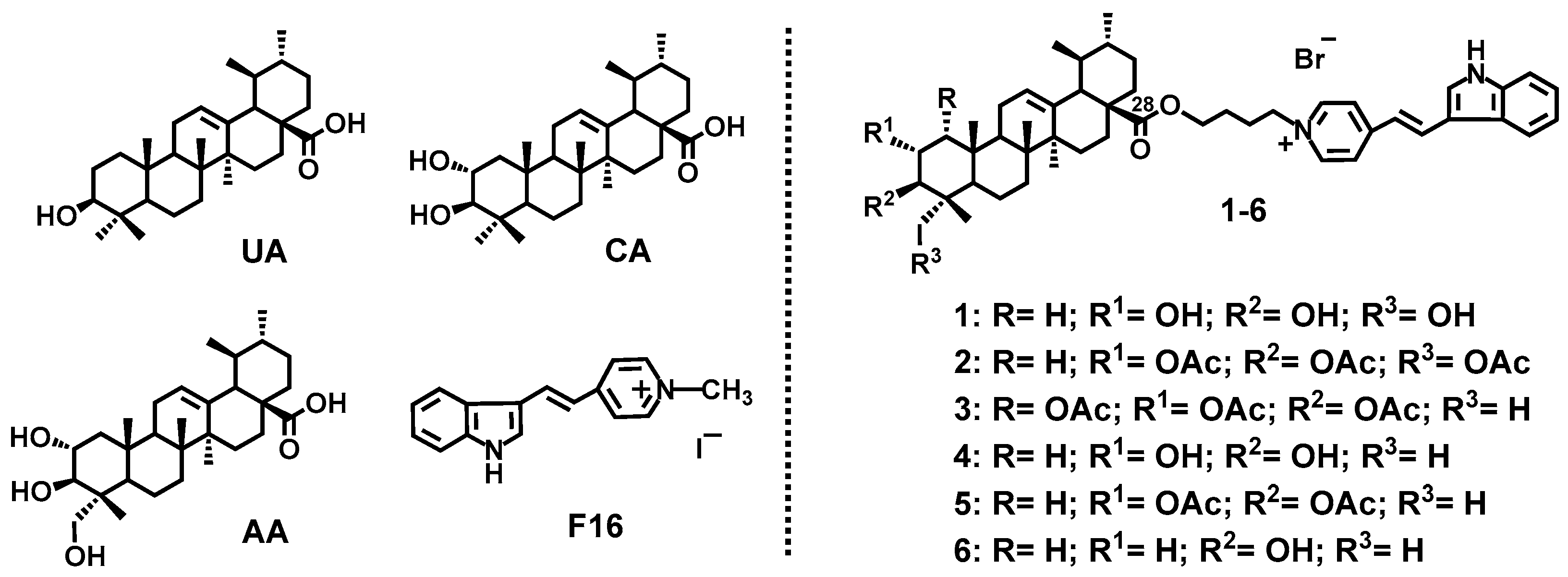
:1. Introduction
2. Materials and Methods
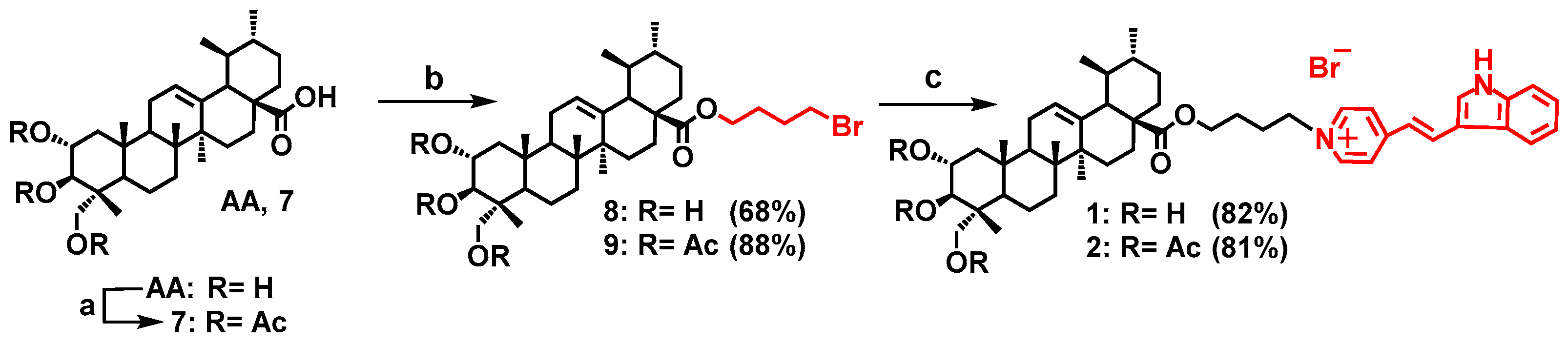
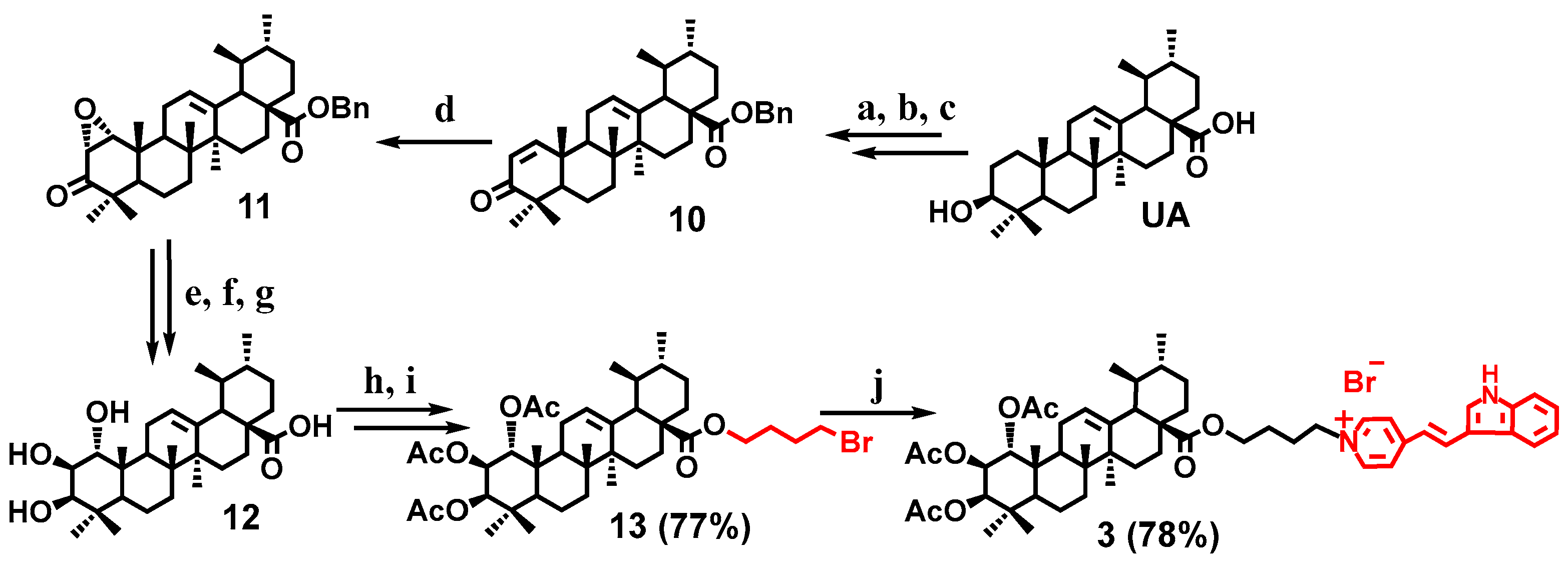
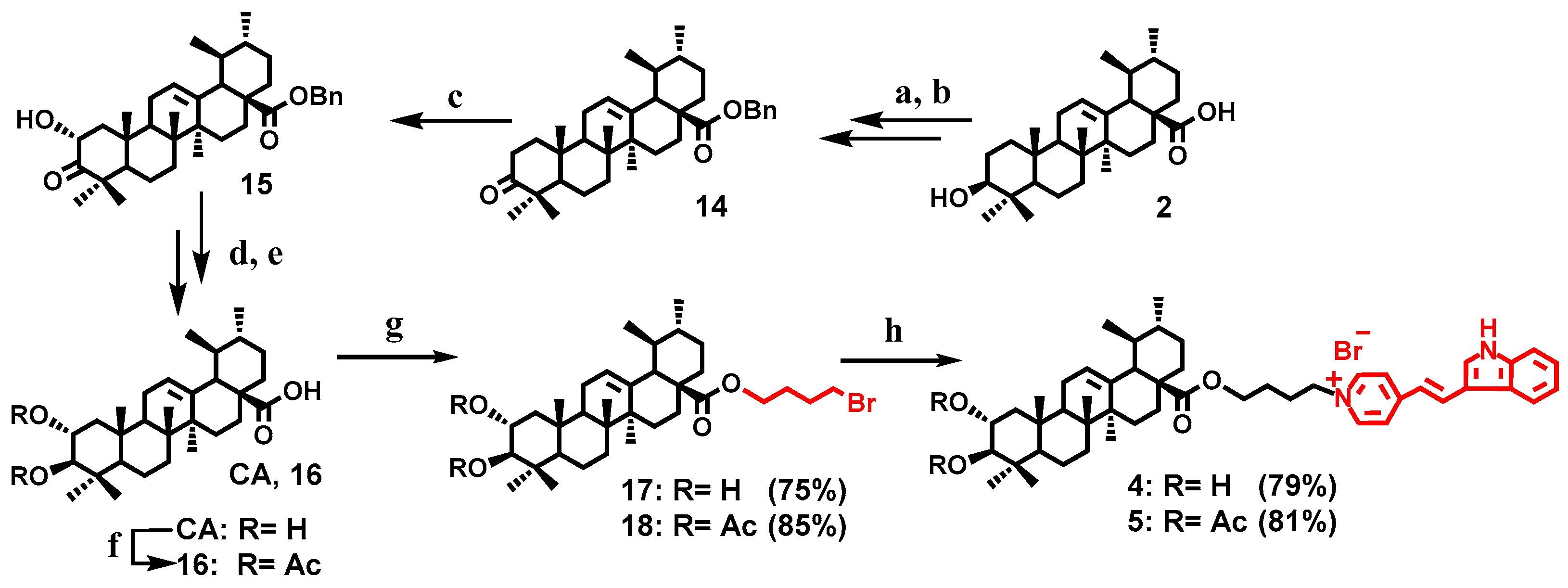
2.1. Chemistry
General Procedure for the Synthesis of the Conjugates 1–5
2.2. Biology
2.2.1. Cell Lines and Culture Conditions
2.2.2. Cytotoxic Assay of Conjugates (MTT Assay)
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, X.; Liu, R.H. Triterpenoids isolated from apple peels have potent antiproliferative activity and may be partially responsible for apple’s anticancer activity. J. Agric. Food Chem. 2007, 55, 4366–4370. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, L.; Szakiel, A.; Głowacka, A.; Rozpara, E.; Marszałek, K.; Skąpska, S. Triterpenoids of three apple cultivars–biosynthesis, antioxidative and anti-inflammatory properties, and fate during processing. Molecules 2023, 28, 2584. [Google Scholar] [CrossRef] [PubMed]
- Neto, C.C. Ursolic acid and other pentacyclic triterpenoids: Anticancer activities and occurrence in berries. In Berries and Cancer Prevention; Stoner, G.D., Seeram, N.P., Eds.; Springer Science+Business Media: New York, NY, USA, 2011; pp. 41–49. [Google Scholar] [CrossRef]
- Dzubak, P.; Hajduch, M.; Vydra, D.; Hustova, A.; Kvasnica, M.; Biedermann, D.; Markova, L.; Urbanc, M.; Sarek, J. Pharmacological activities of natural triterpenoids and their therapeutic implications. Nat. Prod. Rep. 2006, 23, 394–411. [Google Scholar] [CrossRef] [PubMed]
- Hussain, H.; Green, I.R.; Ali, I.; Khan, I.A.; Ali, Z.; Al-Sadi, A.M.; Ahmed, I. Ursolic acid derivatives for pharmaceutical use: A patent review (2012–2016). Expert. Opin. Ther. Pat. 2017, 27, 1061–1072. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Gao, Y.; Wang, A.; Zhou, X.; Zheng, Y.; Zhou, J. Evolution in medicinal chemistry of ursolic acid derivatives as anticancer agents. Eur. J. Med. Chem. 2015, 92, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Dai, X.; Kumar, A.P.; Tan, B.K.; Sethi, G.; Bishayee, A. Ursolic acid in cancer prevention and treatment: Molecular targets, pharmacokinetics and clinical studies. Biochem. Pharmacol. 2013, 85, 1579–1587. [Google Scholar] [CrossRef] [PubMed]
- Van, L.T.; Thi, Q.N.V.; Van, C.T.; Thi, P.T.T.; Thi, N.P.; Tuan, T.N.; Thi, T.H.L.; Thi, N.N.; Thi, T.D.; Van, S.T. Synthesis of asiatic acid derivatives and their cytotoxic activity. Med. Chem. Res. 2018, 27, 1609–1623. [Google Scholar] [CrossRef]
- Xu, Y.; Ge, R.; Du, J.; Xin, H.; Yi, T.; Sheng, J.; Wang, Y.; Ling, C. Corosolic acid induces apoptosis through mitochondrial pathway and caspases activation in human cervix adenocarcinoma HeLa cells. Cancer Lett. 2009, 284, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Spivak, A.Y.; Nedopekina, D.A.; Gubaidullin, R.R.; Davletshin, E.V.; Tukhbatullin, A.A.; D’yakonov, V.A.; Yunusbaeva, M.M.; Dzhemileva, L.U.; Dzhemilev, U.M. Pentacyclic triterpene acid conjugated with mitochondria-targeting cation F16: Synthesis and evaluation of cytotoxic activities. Med. Chem. Res. 2021, 30, 940–951. [Google Scholar] [CrossRef]
- Fantin, V.R.; Berardi, M.J.; Scorrano, L.; Korsmeyer, S.J.; Leder, P. A novel mitochondriotoxic small molecule that selectively inhibits tumor cell growth. Cancer Cell 2002, 2, 29–42. [Google Scholar] [CrossRef]
- Huang, R.-Z.; Jin, L.; Wang, C.-G.; Xu, X.-J.; Du, Y.; Liao, N.; Ji, M.; Liao, Z.X.; Wang, H.-S. A pentacyclic triterpene derivative possessing polyhydroxyl ring A suppresses growth of HeLa cells by reactive oxygen species-dependent NF-κB pathway. Eur. J. Pharmacol. 2018, 838, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Luo, H.; Li, Q.; Wang, D.; Zhang, J.; Hao, X.; Yang, X. Pentacyclic triterpene derivatives possessing polyhydroxyl ring A inhibit Gram-positive bacteria growth by regulating metabolism and virulence genes expression. Eur. J. Med. Chem. 2015, 95, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-H.; Chen, M.-C.; Liu, F.; Xu, Z.; Tian, X.-T.; Xie, Y.; Huang, C.-G. Synthesis and cytotoxic activity of novel C-23-modified asiatic acid derivatives. Molecules 2020, 25, 3709. [Google Scholar] [CrossRef] [PubMed]
- Kraft, O.; Hartmann, A.-K.; Brandt, S.; Hoenke, S.; Heise, N.V.; Csuk, R.; Mueller, T. Asiatic acid as a leading structure for derivatives combining sub-nanomolar cytotoxicity, high selectivity, and the ability to overcome drug resistance in human preclinical tumor models. Eur. J. Med. Chem. 2023, 250, 115189. [Google Scholar] [CrossRef] [PubMed]
- Heise, N.; Becker, S.; Mueller, T.; Bache, M.; Csuk, R.; Guttler, A. Mitochondria-targeting 1,5-diazacyclooctane-spacered triterpene rhodamine conjugates exhibit cytotoxicity at sub-nanomolar concentration against breast cancer cells. Int. J. Mol. Sci. 2023, 24, 10695. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.T.; Camelio, A.M.; Claussen, K.R.; Cho, J.; Tremmel, L.; DiGiovanni, J.; Siegel, D. Synthesis of oxygenated oleanolic and ursolic acid derivatives with anti-inflammatory properties. Bioorg Med. Chem. Lett. 2015, 25, 4342–4346. [Google Scholar] [CrossRef] [PubMed]
- Dubinin, M.V.; Nedopekina, D.A.; Ilzorkina, A.I.; Semenova, A.A.; Sharapov, V.A.; Davletshin, E.V.; Mikina, N.V.; Belsky, Y.P.; Spivak, A.Y.; Akatov, V.S.; et al. Conjugation of triterpenic acids of ursane and oleanane types with mitochondria-targeting cation F16 synergistically enhanced their cytotoxicity against tumor cells. Membranes 2023, 13, 563. [Google Scholar] [CrossRef] [PubMed]
- Spivak, A.Y.; Nedopekina, D.A.; Davletshin, E.V.; Shuvalov, O.I.; Kirdeeva, Y.N.; Belsky, Y.P. Synthesis and cytotoxic evaluation of F16 derivatives pentacyclic triterpenoid acids possessing a polyhydroxylated ring A. Preprint 2022. [Google Scholar] [CrossRef]
| No. | H1299 | A549 | MEF | SI 1 | SI 2 |
|---|---|---|---|---|---|
| 1 | 13.51 ± 1.81 | 9.03 ± 0.59 | 20.04 ± 6.73 | 1.48 | 2.21 |
| 2 | 8.51 ± 2.05 | 3.80 ± 1.86 | 5.85 ± 0.22 | 0.68 | 1.54 |
| 3 | 3.57 ± 0.20 | 4.81 ± 2.15 | 3.79 ± 0.32 | 1.06 | 0.79 |
| 4 | 4.01 ± 1.69 | 1.87 ± 0.14 | 6.76 ± 1.58 | 1.69 | 3.62 |
| 5 | 3.58 ± 0.30 | 3.04 ± 0.34 | 5.79 ± 0.49 | 1.62 | 1.91 |
| 6 | 2.80 ± 0.25 | 2.40 ± 0.30 | n.d. | – | – |
| UA | 97.60 ± 5.63 | 68.93 ± 19.63 | 50.39 ± 16.92 | 0.51 | 0.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spivak, A.; Nedopekina, D.; Davletshin, E. Synthesis and Cytotoxic Activity of Conjugates of Mitochondrial-Directed Cationic Compound F16 with Ursane-Structure Triterpenic Acids Containing a Polyhydroxylated A-ring. Chem. Proc. 2023, 14, 43. https://doi.org/10.3390/ecsoc-27-16177
Spivak A, Nedopekina D, Davletshin E. Synthesis and Cytotoxic Activity of Conjugates of Mitochondrial-Directed Cationic Compound F16 with Ursane-Structure Triterpenic Acids Containing a Polyhydroxylated A-ring. Chemistry Proceedings. 2023; 14(1):43. https://doi.org/10.3390/ecsoc-27-16177
Chicago/Turabian StyleSpivak, Anna, Darya Nedopekina, and Eldar Davletshin. 2023. "Synthesis and Cytotoxic Activity of Conjugates of Mitochondrial-Directed Cationic Compound F16 with Ursane-Structure Triterpenic Acids Containing a Polyhydroxylated A-ring" Chemistry Proceedings 14, no. 1: 43. https://doi.org/10.3390/ecsoc-27-16177
APA StyleSpivak, A., Nedopekina, D., & Davletshin, E. (2023). Synthesis and Cytotoxic Activity of Conjugates of Mitochondrial-Directed Cationic Compound F16 with Ursane-Structure Triterpenic Acids Containing a Polyhydroxylated A-ring. Chemistry Proceedings, 14(1), 43. https://doi.org/10.3390/ecsoc-27-16177







