The Synthesis of Various 2-Imino-2H-chromene-3-carbonitrile Derivatives †
Abstract
:1. Introduction
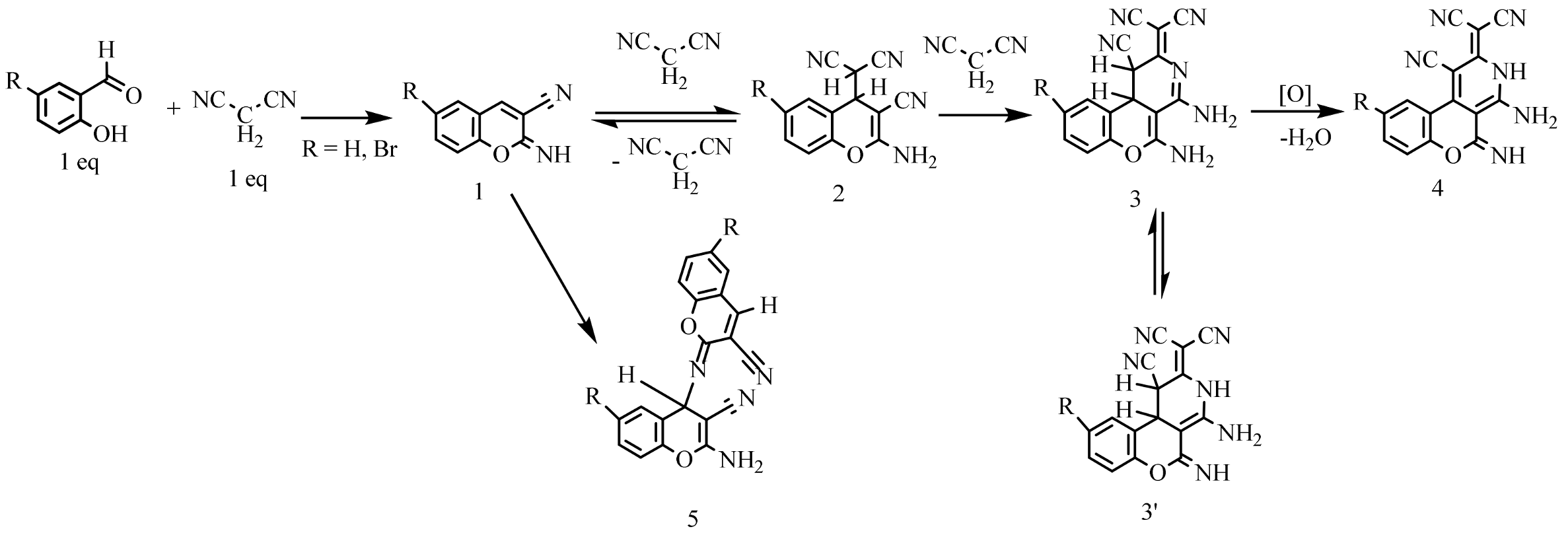
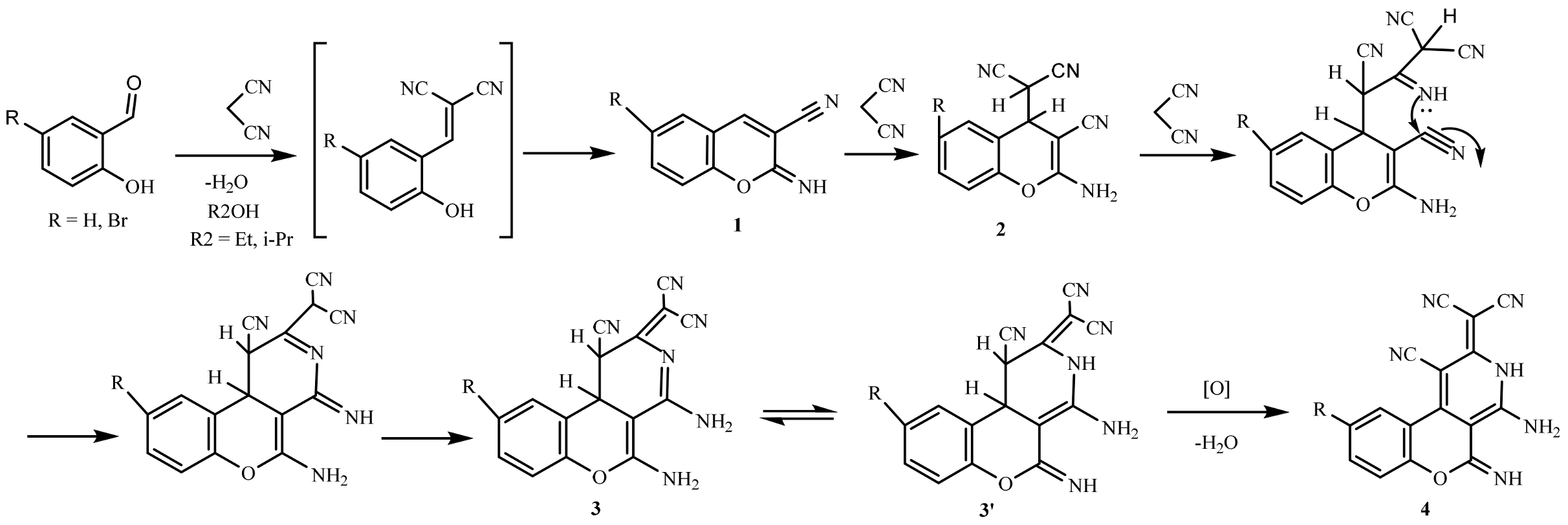
2. Results and Discussion
3. Experimental
3.1. General Information, Instrumentation, and Chemicals
3.2. Synthesis and Characterization of the Compounds
- 2-(4,5-diamino-1-cyano-2H-chromeno[3,4-c]pyridin-2-ylidene)malononitrile 3a and 2-(4-amino-1-cyano-5-imino-1,3,5,10b-tetrahydro-2H-chromeno[3,4-c]pyridin-2-ylidene)malononitrile 3a′
- (A) Equimolar amounts of malononitrile (0.13 g, 0.002 mol) and salicylic aldehyde (0.002 mol) were refluxed in dioxane for 6 h. The beige crystals that precipitated were filtered off, washed with hexane, and dried in desiccators. (B) 2a (0.3 g) was stirred in IPA at 60 °C for 1 h. The beige crystals that precipitated were filtered off, washed with hexane, and dried in desiccators. (C) 2a (0.3 g) was stirred in dioxane in an ultrasonic bath at room temperature for 1 h. The crystals that precipitated were filtered off, washed with hexane, and dried in desiccators.
- M.p. = 287–288 °C. Found, %; C, 62.37; H, 3.11; N, 27.46. C16H10N6O. Calculated, %: C, 63.57; H, 3.33; N, 27.80; O, 5.29. beige crystals. 1H NMR (CDCl3), δ, ppm: (3a): 4.83 (H1, d, 1H. J = 3.6 Hz), 4.91 (H10b, d, 1H. J = 3.6 Hz), 6.69 (-NH2, s, 2H), 7.08 (-NH2, s, 2H), 7.20 (H7, d, 1H. J = 8 Hz), 7.23–7.28 (H8-H10, m, 3H). (3a’): 4.58 (H1, d, 1H. J = 3.6 Hz), 5.05 (H10b, d, 1H. J = 3.6 Hz), 7.12 (H7, d, 1H. J = 8 Hz), 7.41 (H8-H9, t, 2H. J = 8 Hz), 7.46 (H10, d, 1H. J = 8 Hz), 7.51 (-NH2, s, 2H), 8.37 (=NH, s, 1H), 8.85 (=NH, s, 1H). Yield: 70% (A), 86% (B), 87% (C).
- 2-(4,5-diamino-9-bromo-1-cyano-2H-chromeno[3,4-c]pyridin-2-ylidene)malononitrile 3b and 2-(4-amino-9-bromo-1-cyano-5-imino-1,3,5,10b-tetrahydro-2H-chromeno[3,4-c]pyridin-2-ylidene)malononitrile 3b′
- (A) Equimolar amounts of malononitrile (0.13 g, 0.002 mol) and salicylic aldehyde (0.002 mol) were refluxed in IPA in the presence of Et3N (3 drops) for 6 h. The brown crystals that precipitated were filtered off, washed with hexane, and dried in desiccators. (B) 2b (0.35 g) in IPA was refluxed for 4 h. After cooling, the crystalline solid was filtered off, washed with hexane, and dried in desiccators. (C) 2b (0.3 g) was stirred in dioxane in ultrasonic bath at room temperature for 2 h. Brown crystals that precipitated were filtered off, washed with hexane, and dried in desiccators.
- M.p. = 280–282 °C. Brown crystals. Calculated, %: C, 50.41; H, 2.38; Br, 20.96; N, 22.05; O, 4.20. C16H9BrN6O. Found, %: C, 50.47; H, 2.87; N, 22.52. 1H NMR (DMSO-d6), δ, ppm: 4.8–4.9 (H1-H10b, dd, 2H. J = 4 Hz) (3b): 7.1 (=NH, s, 1H), 6.72 (-NH2, s, 2H), 7.64–7.61 (H8, d, 1H. J = 8 Hz), 7.5 (H10, s, 1H), 7.21–7.19 (H7, d, 1H. J = 8 Hz); (3b’): 3.65 (=NH, s, 1H), 6.34 (=NH, s, 1H), 6.53 (=NH, s, 1H), 6.97–6.95 (H7, d, 1H. J = 8 Hz), 7.38–7.35 (H8, d, 1H. J = 8 Hz), 7.29 (H10, s, 1H). Yield: 76% (A), 78% (B), 85% (C).
- 2-(4-amino-1-cyano-5-imino-3,5-dihydro-2H-chromeno[3,4-c]pyridin-2-ylidene)malononitrile 4a
- (A) Equimolar amounts of malononitrile (0.13 g, 0.002 mol) and salicylic aldehyde (0.002 mol) were stirred in H2O-PEG-400 solution at 40 °C for 4 h The orange-brown crystals that precipitated were filtered off, washed with hexane, and dried in air. (B) 2a (0.35 g) in IPA was refluxed for 4 h. After cooling, the crystalline solid was filtered off and dried in air.
- M.p. = 250–252 °C. Orange-brown crystals. Calculated, %: C, 64.00; H, 2.69; N, 27.99; O, 5.33. C16H8N6O. Found, %: C, 63.76.00; H, 2.99; N, 28.05. 1H NMR (DMSO-d6), δ, ppm: 3.65 (=NH, s, 1H), 6.31 (=NH, s, 2H), 6.50 (-NH2, s, 2H), 6.98 (H10, d, 1H. J = 8 Hz), 7.06 (H9, t, 1H. J = 8 Hz), 7.54 (H7, d, 1H. J = 8 Hz), 7.79 (H8, t, 1H. J = 8 Hz). 1H/13C HSQC (DMSO-d6), δ, ppm: 6.98/116.74 (H10/C10), 7.06/124.23 (H9/C9), 7.54/125.79 (H7/C7), 7.79/134.80 (H8/C8). 1H/13C HMBC (DMSO-d6), δ, ppm: 3.65/86.05 (=NH/C1), 3.65/119.48 (=NH/C10a), 3.65/151.08 (=NH/C4), 3.66/168.96 (=NH/C5), 6.52/70.54 (-NH2/C4a), 6.52/86.05, (-NH2/C1),6.31/70.54 (=NH/C4a). Yield: 84% (A), 75% (B).
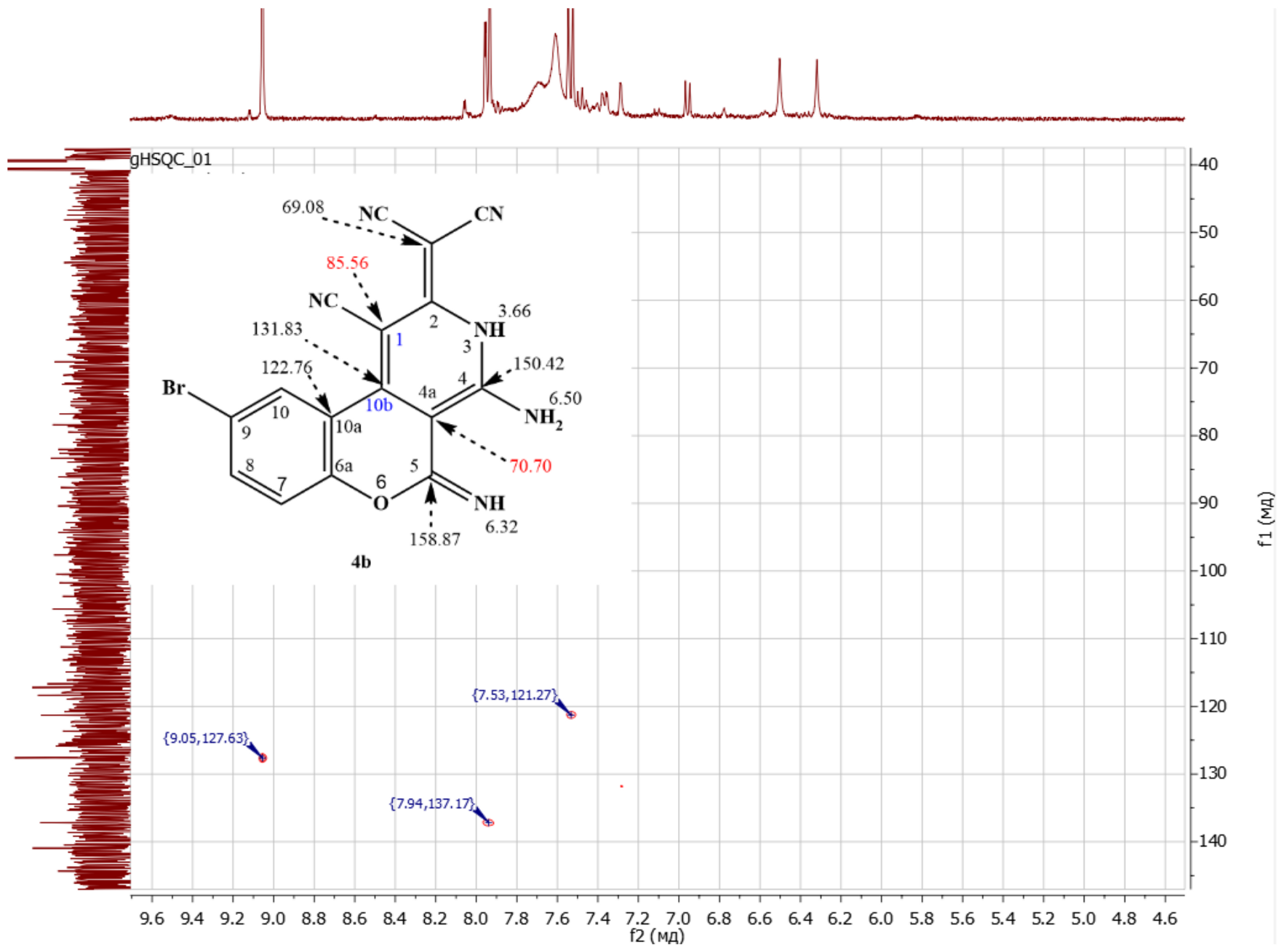
- 2-(4-amino-9-bromo-1-cyano-5-imino-3,5-dihydro-2H-chromeno[3,4-c]pyridin-2-ylidene)malononitrile 4b
- (A) Equimolar amounts of malononitrile (0.13 g, 0.002 mol) and 5-bromsalicylic aldehyde (0.002 mol) were refluxed in IPA for 6 h. After cooling, the crystalline solid was filtered off and dried in air. (B) 2b (0.35 g) in IPA was refluxed for 5 h. The crystalline solid was filtered off and dried in air. (C) 2b (0.35 g) was stirred in THF at 40 °C for 5 h. The crystalline solid was filtered off and dried in air.
- M.p. = 270–272 °C. Brown crystals. Calculated, %: C, 50.68; H, 1.86; Br, 21.07; N, 22.16; O, 4.22. C16H7BrN6O. Found, C, %: 50,47; H, 2.05; N, 22.49. 1H NMR (DMSO-d6), δ, ppm: 3.66 (=NH, s, 1H), 6.20 (=NH, s, 2H), 6.50 (-NH2, s, 2H), 7.54 (H7, d, 1H. J = 8 Hz), 7.94 (H8, d, 1H. J = 8 Hz), 9.05 (H10, s, 1H. J = 8 Hz). 1H/13C HSQC (DMSO-d6), δ, ppm: 7.53/121.27 (H7/C7), 7.94/137.17 (H8/C8), 9.05/127.63 (H10/C10). 1H/13C HMBC (DMSO-d6), δ, ppm: 3.66/85.56 57 (=NH/C1). 3.66/122.76 (=NH/C10a), 3.66/131.83 (=NH/C10b), 3.66/150.42 (=NH/C4), 3.66/158.87 (=NH/C5), 6.32/70.70 (=NH/C4a), 6.50/70.70 (-NH2/C4a), 6.50/85.56 (-NH2/C1). Yield: 70% (A), 75% (B), 86% (C).
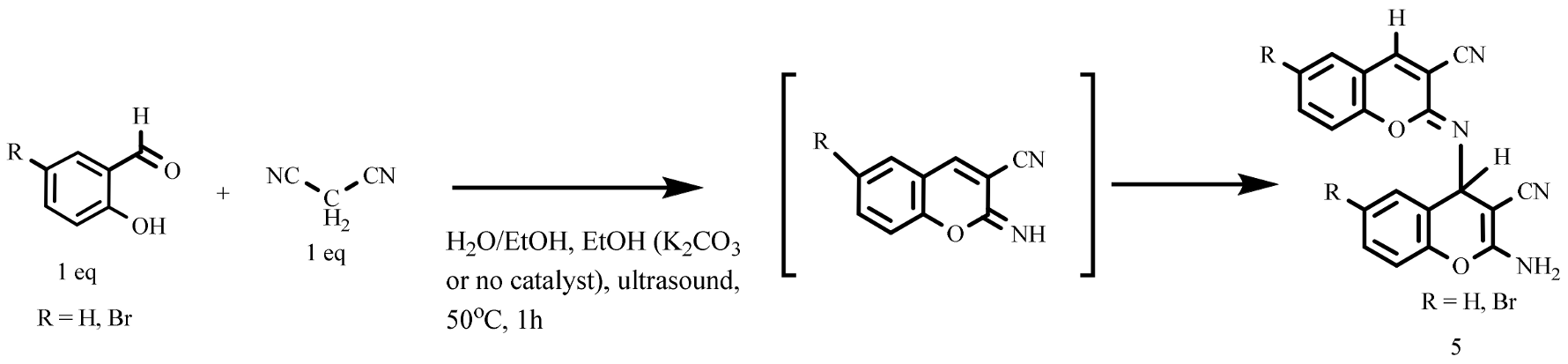
- 2-amino-6-R-4-((6-R-3-cyano-2H-chromen-2-ylidene)amino)-4H-chromene-3-carbonitriles 5b
- (A) Equimolar amounts of malononitrile (0.13 g, 0.002 mol) and 5-bromsalicylic aldehyde (0.002 mol) were heated in ethanol in an ultrasonic bath at 55 °C for 1 h. The beige crystals that precipitated were filtered off, washed with hexane, and dried in a desiccator. (B) Equimolar amounts of malononitrile (0.13 g, 0.002 mol) and 5-bromsalicylic aldehyde (0.002 mol) were heated in aqueous–ethanolic medium (1:1) in the presence of potassium carbonate (3 mol %) in an ultrasonic bath at 55 °C for 1 h. The beige crystals were filtered off, washed with hexane, and dried in a desiccator.
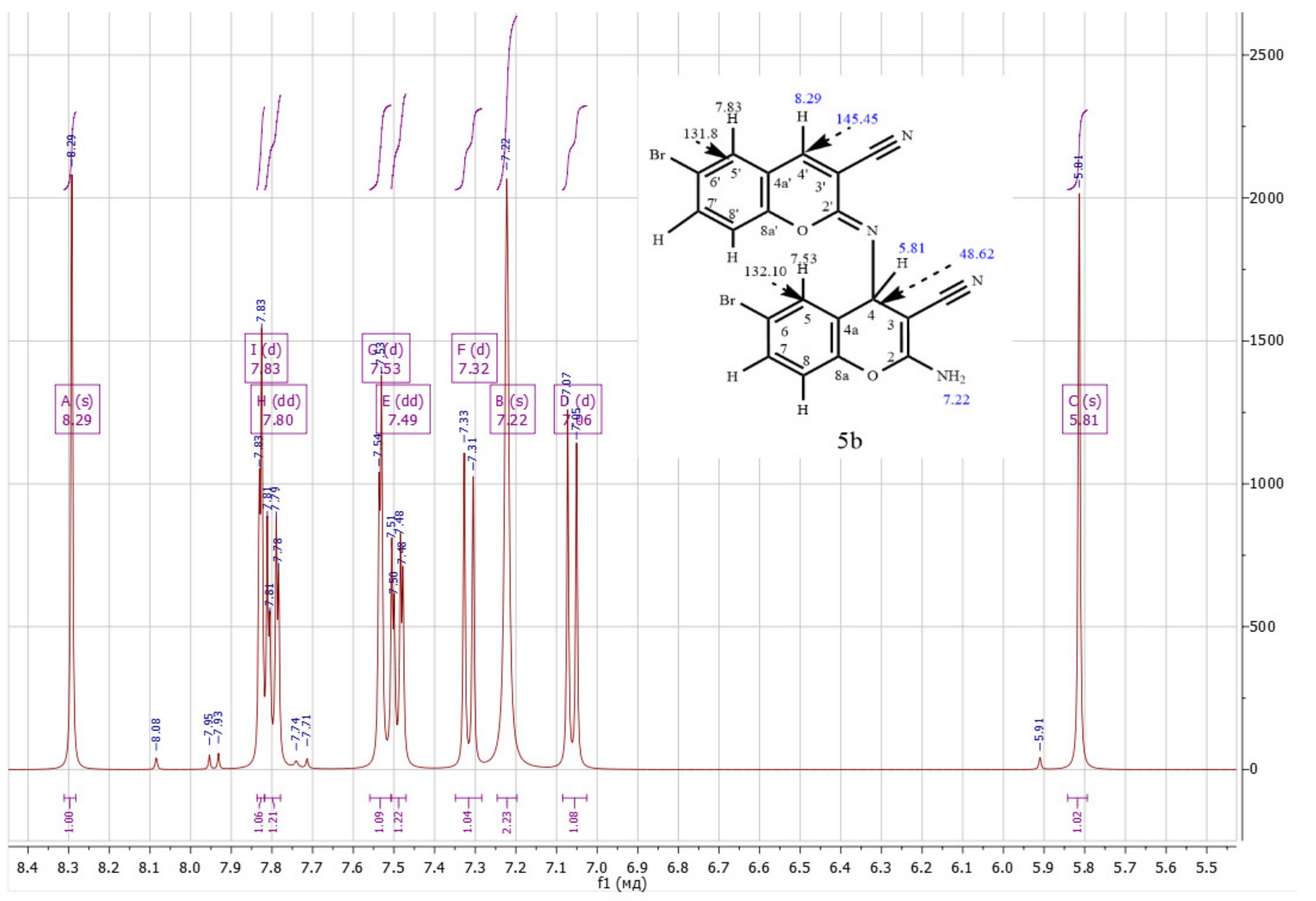
- M.p. = 200–201 °C. Beige crystals. Calculated, %: C, 48.22; H, 2.02; Br, 32.08; N, 11.25; O, 6.42. C20H10Br2N4O2. Found, %: C, 47.98; H, 2.07; N, 11.85. 1H NMR (DMSO-d6), δ, ppm: 5.81 (H4, s, 1H), 7.06 (H8, d, 1H. J = 8 Hz), 7.22 (-NH2, s, 2H), 7.31 (H8′, d, 1H. J = 8 Hz), 7.49 (H7, d, 1H. J = 8 Hz), 7.53 (H5, s, 1H), 7.78–7.81 (H7′, d, 1H. J = 8 Hz), 7.83 (H5′, s, 1H), 8.29 (H4′, s, 1H). 1H/13C HSQC (DMSO-d6), δ, ppm: 5.81/48.62 (H4/C4), 7.07/118.80 (H8/C8), 7.32/118.68 (H8′/C8′), 7.49/132.22 (H7/C7), 7.53/132.10 (H5/C5), 7.80/136.76 (H7′/C7′),7.83/131.80 (H5′/C5′), 8.29/145.45 (H4′/C4′). 1H/13C HMBC (DMSO-d6), δ, ppm: 5.81/54.90 (H4/C3), 5.81/120.47 (H4/-CN), 5.81/124.94 (H4/C4a), 5.81/132.06 (H4/C5), 5.81/146.44 (H4/C2′), 5.81/148.48 (H4/C8a), 5.81/162.07 (H4/C2), 7.22/54.89 (-NH2/C3), 7.53/48.65 (H5/C4), 7.83/145.53 (H5′/C4′), 7.83/152.63 (H5′/C8a’), 8.29/115.16 (H4′/-CN’), 8.29/131.75 (H4′/C5′), 8.29/146.43 (H4′/C2′), 8.29/152.62 (H4′/C8a’). Yield: 80% (A), 83% (B).
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salehian, F.; Nadri, H.; Jalili-Baleh, L.; Youseftabar-Miri, L.; Bukhari SN, A.; Foroumadi, A.; Küçükkilinç, T.T.; Sharifzadeh, M.; Khoobi, M. A review: Biologically active 3, 4-heterocycle-fused coumarins. Eur. J. Med. Chem. 2021, 212, 113034. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Dias, T.A.; Brito, A.; Proença, F. Biological importance of structurally diversified chromenes. Eur. J. Med. Chem. 2016, 123, 487–507. [Google Scholar] [CrossRef] [PubMed]
- Patra, P. A concise review on pyridocoumarin/azacoumarin derivatives: Synthesis and biological activity. ChemistrySelect 2019, 4, 2024–2043. [Google Scholar] [CrossRef]
- Costa, M.; Areias, F.; Abrunhosa, L.; Venâncio, A.; Proença, F. The condensation of salicylaldehydes and malononitrile revisited: Synthesis of new dimeric chromene derivatives. J. Org. Chem. 2008, 73, 1954–1962. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, C.N.; McMurry TB, H.; O’Brien, J.E.; Draper, S.M. Reactions of2-Oxo-2H-1-benzopyran-3-carbonitrile. J. Chem. Res. Synop. 1997, 312–313. [Google Scholar] [CrossRef]
- Yang, F.; Wang, Z.; Wang, H.; Wang, C.; Wang, L. An efficient condensation of substituted salicylaldehyde and malononitrile catalyzed by lipase under microwave irradiation. RSC Adv. 2015, 5, 57122–57126. [Google Scholar] [CrossRef]
- Smuszkiewicz, A.; Lopez-Sanz, J.; Sobczak, I.; Martin-Aranda, R.M.; Ziolek, M.; Perez-Mayoral, E. Tantalum vs. Niobium MCF nanocatalysts in the green synthesis of chromene derivatives. Catal. Today 2019, 325, 47–52. [Google Scholar] [CrossRef]
- De Abrantes, P.G.; de Abrantes, P.G.; dos Santos Silva, D.A.; Magalhães, R.R.; da Silva PB, N.; Militão GC, G.; de Menezes, R.P.B.; Scotti, L.; Scotti, M.T.; Vale, J.A. Synthesis of 2-amino-4H-chromenes catalyst-free via sequential Knoevenagel-Michael reaction and evaluation of biological activity in tumor cells. Med. Chem. Res. 2023, 32, 2234–2244. [Google Scholar] [CrossRef]
- Yadav, S.; Srivastava, M.; Rai, P.; Singh, J.; Tiwari, K.P.; Singh, J. Visible light induced, catalyst free, convenient synthesis of chromene nucleus and its derivatives using water–ethanol mixture as a solvent. New J. Chem. 2015, 39, 4556–4561. [Google Scholar] [CrossRef]
- O’Callaghan, C.N.; McMurry TB, H.; O’Brien, J.E. Synthetic reactions of 2-(2-amino-3-cyano-4 H-[1] benzopyran-4-yl) propane-1, 3-dinitrile with reactive methylene compounds. J. Chem. Soc. Perkin Trans. 1 1995, 417–420. [Google Scholar] [CrossRef]
- Elinson, M.N.; Gorbunov, S.V.; Vereshchagin, A.N.; Nasybullin, R.F.; Goloveshkin, A.S.; Bushmarinov, I.S.; Egorov, M.P. Chemical and electrocatalytic cascade cyclization of salicylaldehyde with three molecules of malononitrile:‘One-pot’simple and efficient way to the chromeno [2,3-b] pyridine scaffold. Tetrahedron 2014, 70, 8559–8563. [Google Scholar] [CrossRef]
- Costa, M.; Areias, F.; Castro, M.; Brea, J.; Loza, M.I.; Proença, F. Synthesis of novel chromene scaffolds for adenosine receptors. Org. Biomol. Chem. 2011, 9, 4242–4249. [Google Scholar] [CrossRef]
- Elinson, M.N.; Dorofeev, A.S.; Miloserdov, F.M.; Ilovaisky, A.I.; Feducovich, S.K.; Belyakov, P.A.; Nikishin, G.I. Catalysis of Salicylaldehydes and Two Different C—H Acids with Electricity: First Example of an Efficient Multicomponent Approach to the Design of Functionalized Medicinally Privileged 2-Amino-4H-Chromene Scaffold. Adv. Synth. Catal. 2008, 350, 591–601. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meshcheryakova, A.A.; Konstantinova, E.A.; Melkonyan, K.A.; Khrustaleva, A.A.; Sorokin, V.V. The Synthesis of Various 2-Imino-2H-chromene-3-carbonitrile Derivatives. Chem. Proc. 2023, 14, 42. https://doi.org/10.3390/ecsoc-27-16125
Meshcheryakova AA, Konstantinova EA, Melkonyan KA, Khrustaleva AA, Sorokin VV. The Synthesis of Various 2-Imino-2H-chromene-3-carbonitrile Derivatives. Chemistry Proceedings. 2023; 14(1):42. https://doi.org/10.3390/ecsoc-27-16125
Chicago/Turabian StyleMeshcheryakova, Anna A., Ekaterina A. Konstantinova, Karina A. Melkonyan, Alexandra A. Khrustaleva, and Vitaliy V. Sorokin. 2023. "The Synthesis of Various 2-Imino-2H-chromene-3-carbonitrile Derivatives" Chemistry Proceedings 14, no. 1: 42. https://doi.org/10.3390/ecsoc-27-16125
APA StyleMeshcheryakova, A. A., Konstantinova, E. A., Melkonyan, K. A., Khrustaleva, A. A., & Sorokin, V. V. (2023). The Synthesis of Various 2-Imino-2H-chromene-3-carbonitrile Derivatives. Chemistry Proceedings, 14(1), 42. https://doi.org/10.3390/ecsoc-27-16125





