The Impact of Cast Walker Design on Metabolic Costs of Walking and Perceived Exertion
Abstract
1. Introduction
2. Materials and Methods
3. Results
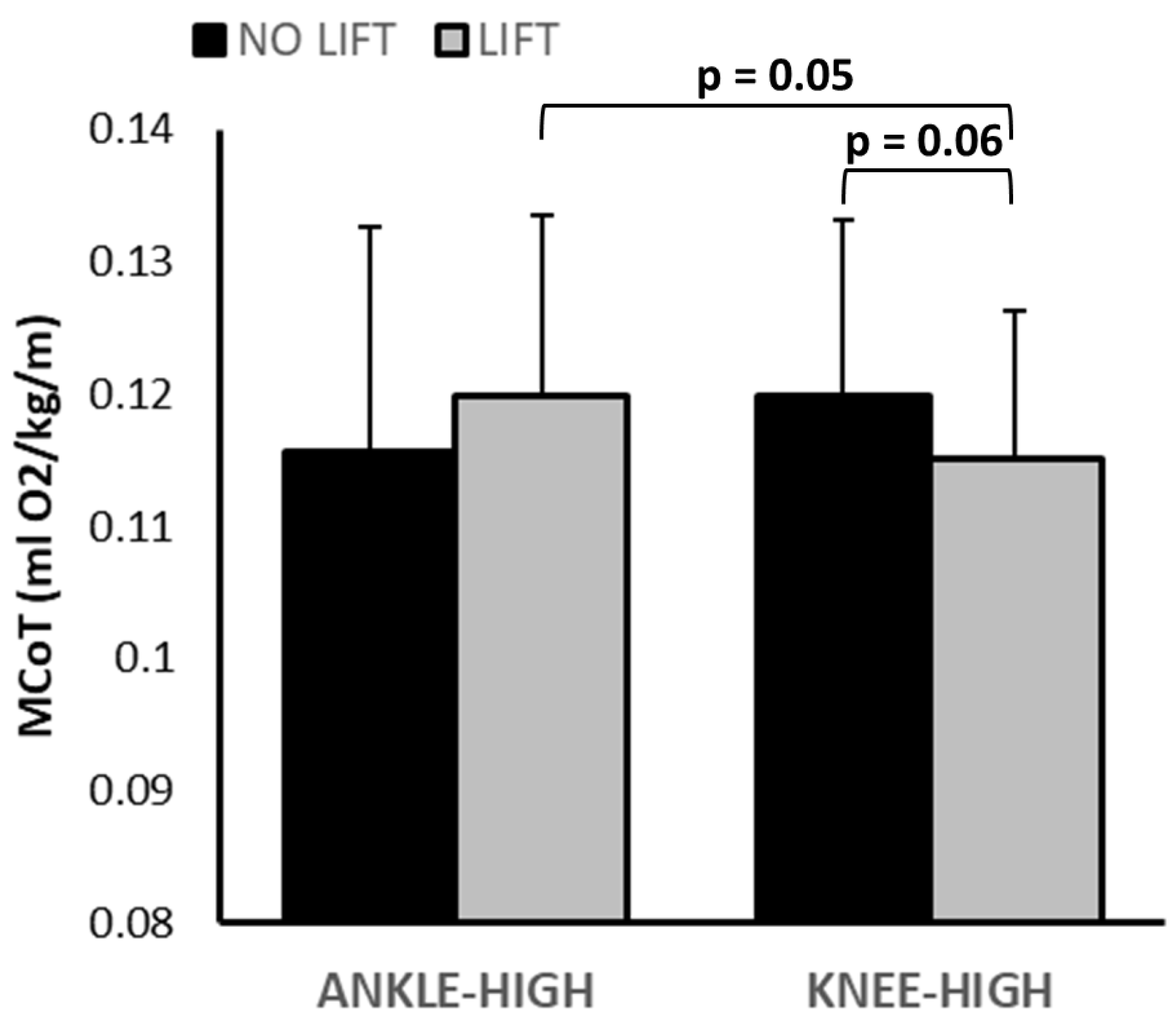
3.1. Metabolic Data
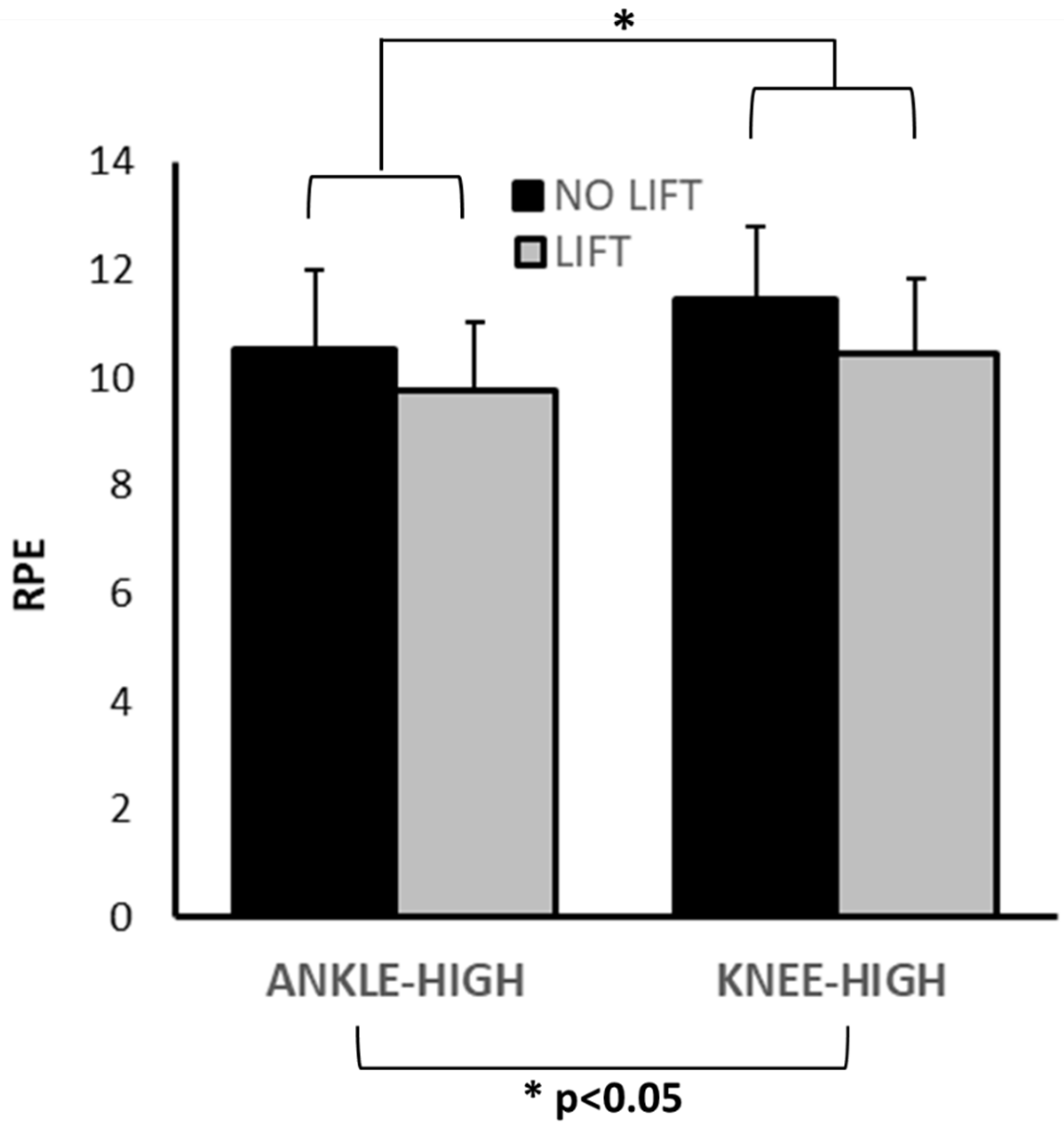
3.2. RPE
3.3. Spatiotemporal Kinematics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CW | cast walker |
| DFU | diabetic foot ulcer |
| LLD | limb length discrepancy |
| MCoT | metabolic cost of transport (MCoT) |
| RMEE | rate of metabolic energy expenditure |
| RPE | rating of perceived exertion (using the Borg scale) |
| VO2 | volumetric oxygen consumption |
References
- Armstrong, D.G.; Boulton, A.J.; Bus, S.A. Diabetic foot ulcers and their recurrence. N. Engl. J. Med. 2017, 376, 2367–2375. [Google Scholar] [CrossRef]
- Pemayun, T.G.D.; Naibaho, R.M.; Novitasari, D.; Amin, N.; Minuljo, T.T. Risk factors for lower extremity amputation in patients with diabetic foot ulcers: A hospital-based case–control study. Diabet. Foot Ankle 2015, 6, 29629. [Google Scholar]
- Lazzarini, P.A.; Armstrong, D.G.; Crews, R.T.; Gooday, C.; Jarl, G.; Kirketerp-Moller, K.; Viswanathan, V.; Bus, S.A. Effectiveness of offloading interventions for people with diabetes-related foot ulcers: A systematic review and meta-analysis. Diabetes Metab. Res. Rev. 2024, 40, e3650. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.C.; Jensen, J.L.; Weber, A.K.; Robinson, D.E.; Armstrong, D.G. Use of pressure offloading devices in diabetic foot ulcers: Do we practice what we preach? Diabetes Care 2008, 31, 2118–2119. [Google Scholar] [CrossRef] [PubMed]
- Hingorani, A.; LaMuraglia, G.M.; Henke, P.; Meissner, M.H.; Loretz, L.; Zinszer, K.M.; Driver, V.R.; Frykberg, R.; Carman, T.L.; Marston, W.; et al. The management of diabetic foot: A clinical practice guideline by the Society for Vascular Surgery in collaboration with the American Podiatric Medical Association and the Society for Vascular Medicine. J. Vasc. Surg. 2016, 63 (Suppl. 2), 3S–21S. [Google Scholar] [CrossRef] [PubMed]
- Bus, S.A.; Armstrong, D.G.; Crews, R.T.; Gooday, C.; Jarl, G.; Kirketerp-Moller, K.; Viswanathan, V.; Lazzarini, P.A. Guidelines on offloading foot ulcers in persons with diabetes (IWGDF 2023 update). Diabetes Metab. Res. Rev. 2023, 40, e3647. [Google Scholar]
- Raspovic, A.; Landorf, K.B. A survey of offloading practices for diabetes-related plantar neuropathic foot ulcers. J. Foot Ankle Res. 2014, 7, 35. [Google Scholar] [CrossRef]
- Orlando, G.; Balducci, S.; Bazzucchi, I.; Pugliese, G.; Sacchetti, M. Muscle fatigability in type 2 diabetes. Diabetes/Metab. Res. Rev. 2017, 33, e2821. [Google Scholar]
- Regensteiner, J.G.; Sippel, J.; McFarling, E.T.; Wolfel, E.E.; Hiatt, W.R. Effects of non-insulin-dependent diabetes on oxygen consumption during treadmill exercise. Med. Sci. Sports Exerc. 1995, 27, 875–881. [Google Scholar]
- Regensteiner, J.G.; Bauer, T.A.; Reusch, J.E.B.; Brandenburg, S.L.; Sippel, J.M.; Vogelsong, A.M.; Smith, S.; Wolfel, E.E.; Eckel, R.H.; Hiatt, W.R. Abnormal oxygen uptake kinetic responses in women with type II diabetes mellitus. J. Appl. Physiol. 1998, 85, 310–317. [Google Scholar] [CrossRef]
- Gurney, B.; Mermier, C.; Robergs, R.; Gibson, A.; Rivero, D. Effects of limb-length discrepancy on gait economy and lower-extremity muscle activity in older adults. J. Bone Jt. Surg. 2001, 83, 907–915. [Google Scholar] [CrossRef]
- Walsh, M.; Connolly, P.; Jenkinson, A.; O’Brien, T. Leg length discrepancy—An experimental study of compensatory changes in three dimensions using gait analysis. Gait Posture 2000, 12, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Miyagi, I.; Ohta, M.; Tamari, M. Effect of leg length discrepancy on dynamic gait stability. Prog. Rehabil. Med. 2023, 8, 20230013. [Google Scholar] [PubMed]
- Azizan, N.; Basaruddin, K.; Salleh, A.; Sulaiman, A.; Safar, M.; Rusli, W. Leg length discrepancy: Dynamic balance response during gait. J. Healthc. Eng. 2018, 2018, 7815451. [Google Scholar] [PubMed]
- Korontzi, M.; Kafetzakis, I.; Mandalidis, D. Effects of artificially induced leg length discrepancy on treadmill-based walking and running symmetry in healthy college students: A lab-based experimental study. Sensors 2023, 23, 9695. [Google Scholar] [CrossRef]
- Donelan, J.M.; Kram, R.; Kuo, A.D. Mechanical and metabolic determinants of the preferred step width in human walking. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2001, 268, 1985–1992. [Google Scholar]
- Jung, S.-j.; An, D.-h.; Shin, S.-s. The Effects of Simulated Mild Leg Length Discrepancy on Gait Parameters and Trunk Acceleration. Phys. Ther. Korea 2018, 25, 9–18. [Google Scholar] [CrossRef]
- Ellis, R.G.; Howard, K.C.; Kram, R. The metabolic and mechanical costs of step time asymmetry in walking. Proc. R. Soc. B Biol. Sci. 2013, 280, 20122784. [Google Scholar]
- Stenum, J.; Choi, J.T. Disentangling the energetic costs of step time asymmetry and step length asymmetry in human walking. J. Exp. Biol. 2021, 224, jeb242258. [Google Scholar] [CrossRef]
- Mrdjenovich, D.E. Off-loading practices for the wounded foot: Concepts and choices. J. Am. Coll. Certif. Wound Spec. 2010, 2, 73–78. [Google Scholar]
- Stolycia, M.L.; Lunn, D.E.; Wilkins, R.A.; Barnett, C.T.; Walker, J. Walking in a controlled ankle motion (CAM) boot: In-boot measurement of joint kinematics and kinetics. J. Biomech. 2024, 176, 112327. [Google Scholar] [CrossRef] [PubMed]
- Vanderpool, M.T.; Collins, S.H.; Kuo, A.D. Ankle fixation need not increase the energetic cost of human walking. Gait Posture 2008, 28, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Lunn, D.E.; Walker, J. Exploring mechanical work changes in controlled ankle motion (CAM) boot walking: The effects of gait speed and shoe levelling. PLoS ONE 2025, 20, e0321978. [Google Scholar] [CrossRef] [PubMed]
- Houdijk, H.; Pollmann, E.; Groenewold, M.; Wiggerts, H.; Polomski, W. The energy cost for the step-to-step transition in amputee walking. Gait Posture 2009, 30, 35–40. [Google Scholar] [CrossRef]
- Stolycia, M.L.; Lunn, D.E.; Stanier, W.; Walker, J.; Wilkins, R.A. Biomechanical effectiveness of controlled ankle motion boots: A systematic review and narrative synthesis. J. Foot Ankle Res. 2024, 17, e12044. [Google Scholar] [CrossRef]
- Crews, R.T.; Sayeed, F.; Najafi, B. Impact of strut height on offloading capacity of removable cast walkers. Clin. Biomech. 2012, 27, 725–730. [Google Scholar] [CrossRef]
- Miller, J.F.; Stamford, B.A. Intensity and energy cost of weighted walking vs. running for men and women. J. Appl. Physiol. 1987, 62, 1497–1501. [Google Scholar] [CrossRef]
- Bus, S.A.; Armstrong, D.G.; Gooday, C.; Jarl, G.; Caravaggi, C.; Viswanathan, V.; Lazzarini, P.A.; International Working Group on the Diabetic Foot (IWGDF). Guidelines on offloading foot ulcers in persons with diabetes (IWGDF 2019 update). Diabetes Metab. Res. Rev. 2020, 36 (Suppl. 1), e3274. [Google Scholar] [CrossRef]
- Julious, S.A. Sample size of 12 per group rule of thumb for a pilot study. Pharm. Stat. J. Appl. Stat. Pharm. Ind. 2005, 4, 287–291. [Google Scholar] [CrossRef]
- Hertzog, M.A. Considerations in determining sample size for pilot studies. Res. Nurs. Health 2008, 31, 180–191. [Google Scholar] [CrossRef]
- Vaughan, C.L.; O’Malley, M.J. Froude and the contribution of naval architecture to our understanding of bipedal locomotion. Gait Posture 2005, 21, 350–362. [Google Scholar] [CrossRef]
- Borg, G. Borg’s perceived exertion and pain scales. In Human Kinetics; American Psychological Association: Washington, DC, USA, 1998. [Google Scholar]
- Robinson, R.; Herzog, W.; Nigg, B. Use of force platform variables to quantify the effects of chiropractic manipulation on gait symmetry. J. Manip. Physiol. Ther. 1987, 10, 172–176. [Google Scholar]
- Hof, A.; Gazendam, M.; Sinke, W. The condition for dynamic stability. J. Biomech. 2005, 38, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Roemmich, R.T.; Leech, K.A.; Gonzalez, A.J.; Bastian, A.J. Trading symmetry for energy cost during walking in healthy adults and persons poststroke. Neurorehabilit. Neural Repair. 2019, 33, 602–613. [Google Scholar] [CrossRef] [PubMed]
- Scherr, J.; Wolfarth, B.; Christle, J.W.; Pressler, A.; Wagenpfeil, S.; Halle, M. Associations between Borg’s rating of perceived exertion and physiological measures of exercise intensity. Eur. J. Appl. Physiol. 2013, 113, 147–155. [Google Scholar] [CrossRef]
- Marcora, S. Perception of effort during exercise is independent of afferent feedback from skeletal muscles, heart, and lungs. J. Appl. Physiol. 2009, 106, 2060–2062. [Google Scholar] [CrossRef]
- Pandolf, K.B. Psychological and physiological factors influencing perceived exertion. In Physical Work and Effort; Elsevier: Amsterdam, The Netherlands, 1977; pp. 371–383. [Google Scholar]
- Caldwell, L.K.; Laubach, L.L.; Barrios, J.A. Effect of specific gait modifications on medial knee loading, metabolic cost and perception of task difficulty. Clin. Biomech. 2013, 28, 649–654. [Google Scholar] [CrossRef]
- Chiou, S.S.; Turner, N.; Zwiener, J.; Weaver, D.L.; Haskell, W.E. Effect of boot weight and sole flexibility on gait and physiological responses of firefighters in stepping over obstacles. Hum. Factors 2012, 54, 373–386. [Google Scholar] [CrossRef]
- Turner, N.L.; Chiou, S.; Zwiener, J.; Weaver, D.; Spahr, J. Physiological effects of boot weight and design on men and women firefighters. J. Occup. Environ. Hyg. 2010, 7, 477–482. [Google Scholar] [CrossRef]
- Smith, J.D.; Martin, P.E. Effects of prosthetic mass distribution on metabolic costs and walking symmetry. J. Appl. Biomech. 2013, 29, 317–328. [Google Scholar] [CrossRef]
- Carlisle, R.E.; Kuo, A.D. Optimization of energy and time predicts dynamic speeds for human walking. eLife 2023, 12, e81939. [Google Scholar] [CrossRef] [PubMed]
- Assogba, T.; Boulet, S.; Detrembleur, C.; Mahaudens, P. The effects of real and artificial leg length discrepancy on mechanical work and energy cost during the gait. Gait Posture 2018, 59, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Crews, R.T.; Shen, B.-J.; Campbell, L.; Lamont, P.J.; Boulton, A.J.M.; Peyrot, M.; Kirsner, R.S.; Vileikyte, L. Role and determinants of adherence to off-loading in diabetic foot ulcer healing: A prospective investigation. Diabetes Care 2016, 39, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.G.; Lavery, L.A.; Wrobel, J.S.; Vileikyte, L. Quality of life in healing diabetic wounds: Does the end justify the means? J. Foot Ankle Surg. 2008, 47, 278–282. [Google Scholar] [CrossRef]
- Huebschmann, A.G.; Reis, E.N.; Emsermann, C.; Dickinson, L.M.; Reusch, J.E.B.; Bauer, T.A.; Regensteiner, J.G. Women with type 2 diabetes perceive harder effort during exercise than nondiabetic women. Appl. Physiol. Nutr. Metab. 2009, 34, 851–857. [Google Scholar] [CrossRef]
- Julius, L.M.; Brach, J.S.; Wert, D.M.; VanSwearingen, J.M. Perceived effort of walking: Relationship with gait, physical function and activity, fear of falling, and confidence in walking in older adults with mobility limitations. Phys. Ther. 2012, 92, 1268–1277. [Google Scholar] [CrossRef]
- Czerniecki, J.M.; Morgenroth, D.C. Metabolic energy expenditure of ambulation in lower extremity amputees: What have we learned and what are the next steps? Disabil. Rehabil. 2017, 39, 143–151. [Google Scholar] [CrossRef]
- Crews, R.T.; Rosenblatt, N.J. 482-P: Do Varied Off-Loading Modalities Have Differing Impacts Upon Users’ Fall Risk? Diabetes 2022, 71 (Suppl. 1), 482. [Google Scholar] [CrossRef]
- Crews, R.T.; Candela, J. Decreasing an Offloading Device’s Size and Offsetting Its Imposed Limb-Length Discrepancy Lead to Improved Comfort and Gait. Diabetes Care 2018, 41, 1400–1405. [Google Scholar] [CrossRef]
- Leon, A.C.; Heo, M. Sample sizes required to detect interactions between two binary fixed-effects in a mixed-effects linear regression model. Comput. Stat. Data Anal. 2009, 53, 603–608. [Google Scholar] [CrossRef]
- Resende, R.A.; Kirkwood, R.N.; Deluzio, K.J.; Cabral, S.; Fonseca, S.T. Biomechanical strategies implemented to compensate for mild leg length discrepancy during gait. Gait Posture 2016, 46, 147–153. [Google Scholar] [CrossRef]
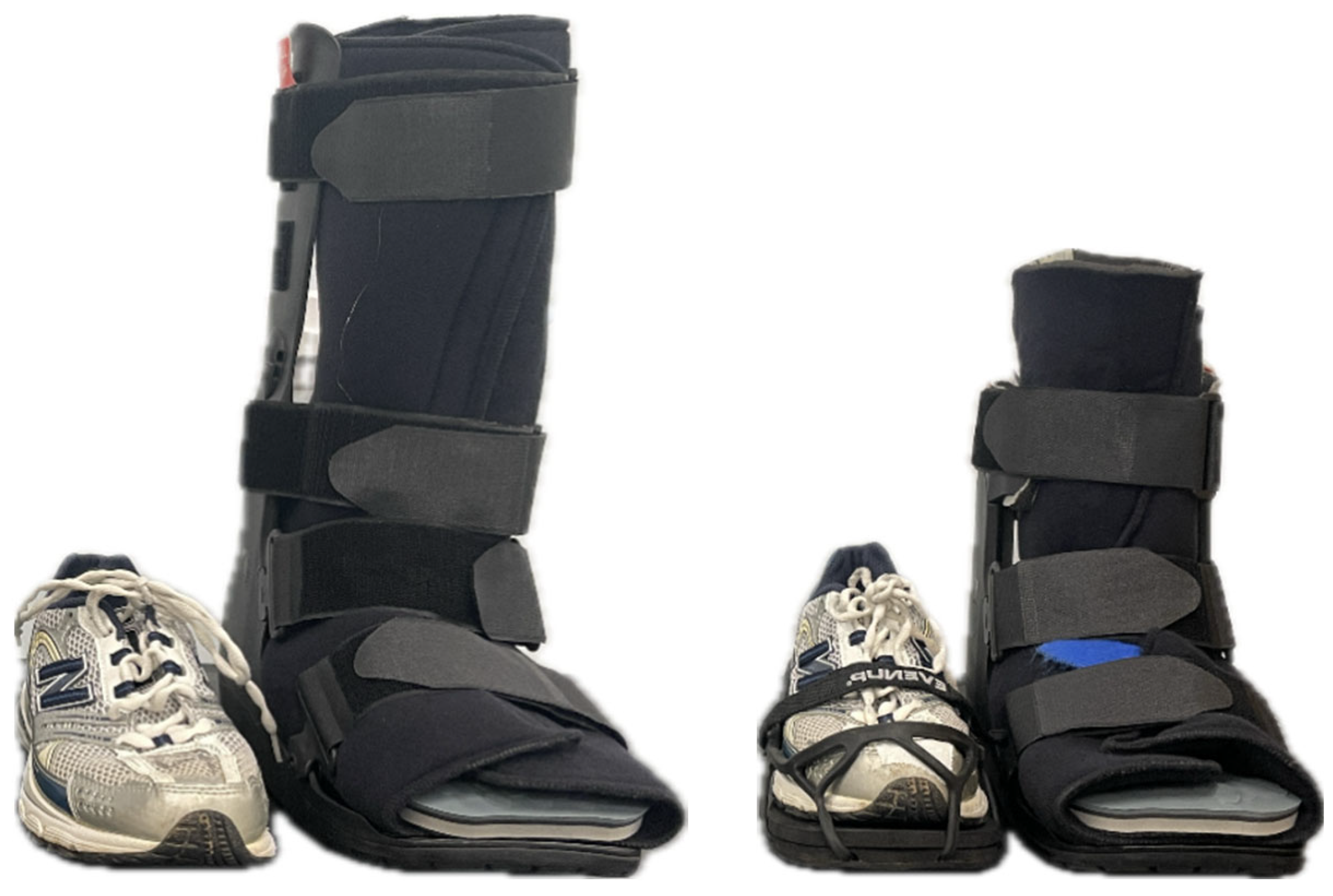

| Outcome | Condition | p-Values | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | Knee-High | Ankle-High | vs. Control | Height | Lift | Lift × Height | ||||
| Lift | No Lift | Lift | No Lift | |||||||
| MCoT (mL/m/kg) | 0.013 ± 0.010 | 0.116 ± 0.010 | 0.122 ± 0.014 | 0.122 ± 0.012 | 0.117 ± 0.012 | KHL: <0.001 KHNL: <0.001 AHL: <0.001 AHNL: <0.001 | 0.66 | 0.70 | 0.02 | NL-KH vs. AH: 0.17 L-KH vs. AH: 0.05 AH-L vs. NL: 0.16 KH–L vs. NL: 0.06 |
| RPE | 8.85 ± 1.21 | 10.46 ± 1.39 | 11.46 ± 1.33 | 9.77 ± 1.24 | 10.54 ± 1.45 | KHL: <0.001 KHNL: <0.001 AHL: 0.003 AHNL: <0.001 | <0.001 | <0.001 | 0.57 | -- |
| SW (cm) | 12.49 ± 1.06 | 16.16 ± 2.36 | 16.56 ± 2.58 | 15.40 ± 2.17 | 15.48 ± 2.55 | KHL: <0.001 KHNL: <0.001 AHL: <0.001 AHNL: <0.001 | 0.03 | 0.63 | 0.70 | -- |
| SL SI | 2.76 ± 1.33 | 8.76 ± 6.56 | 5.18 ± 4.22 | 6.14 ± 4.37 | 6.92 ± 4.54 | KHL: <0.001 KHNL: 0.14 AHL: 0.04 AHNL: 0.008 | 0.88 | 0.27 | 0.04 | NL-KH vs. AH: 0.08 L-KH vs. AH: 0.22 AH-L vs. NL: 0.94 KH–L vs. NL: 0.053 |
| ST SI | 2.77 ± 3.15 | 4.71 ± 3.32 | 2.43 ± 2.39 | 4.74 ± 3.71 | 3.44 ± 2.81 | KHL: 0.08 KHNL: 0.69 AHL: 0.07 AHNL: 0.57 | 0.42 | 0.01 | 0.50 | -- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Standage, E.; Tookey, D.; Ukachukwu, U.; Avalos, M.; Crews, R.T.; Rosenblatt, N.J. The Impact of Cast Walker Design on Metabolic Costs of Walking and Perceived Exertion. Diabetology 2025, 6, 98. https://doi.org/10.3390/diabetology6090098
Standage E, Tookey D, Ukachukwu U, Avalos M, Crews RT, Rosenblatt NJ. The Impact of Cast Walker Design on Metabolic Costs of Walking and Perceived Exertion. Diabetology. 2025; 6(9):98. https://doi.org/10.3390/diabetology6090098
Chicago/Turabian StyleStandage, Emily, Dylan Tookey, Uchechukwu Ukachukwu, Marco Avalos, Ryan T. Crews, and Noah J. Rosenblatt. 2025. "The Impact of Cast Walker Design on Metabolic Costs of Walking and Perceived Exertion" Diabetology 6, no. 9: 98. https://doi.org/10.3390/diabetology6090098
APA StyleStandage, E., Tookey, D., Ukachukwu, U., Avalos, M., Crews, R. T., & Rosenblatt, N. J. (2025). The Impact of Cast Walker Design on Metabolic Costs of Walking and Perceived Exertion. Diabetology, 6(9), 98. https://doi.org/10.3390/diabetology6090098






