Wearable Activity Trackers to Improve Physical Activity and Cardiovascular Risk in Type 2 Diabetes: A Randomized Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
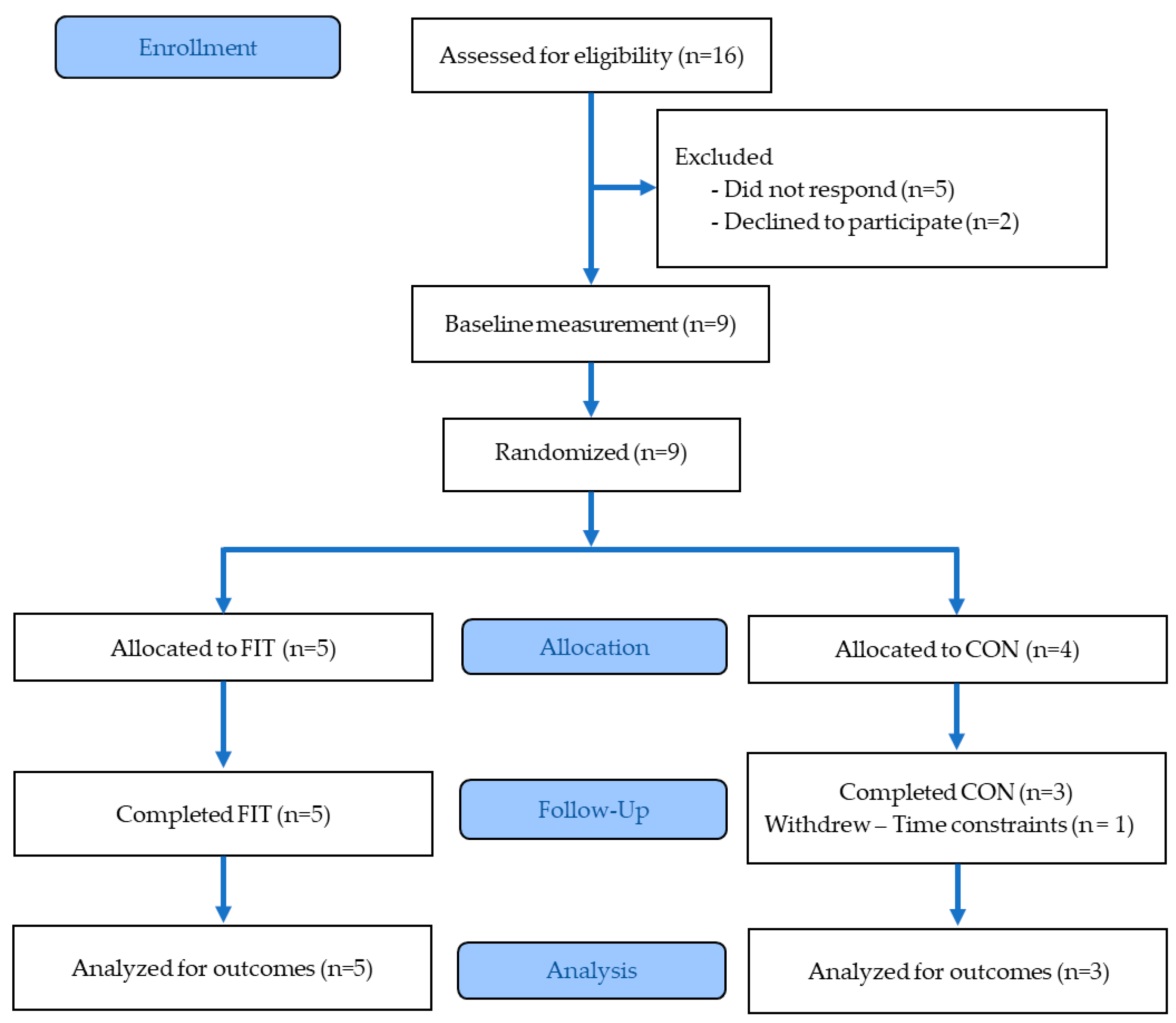
2.2. Participants
2.3. Intervention
2.4. Outcome Measures
2.4.1. Primary Outcomes
2.4.2. Secondary Outcomes
2.5. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Physical Activity Outcomes
3.3. Cardiovascular Health Outcomes
3.4. Metabolic and Anthropometric Outcomes
3.5. Associations Between Physical Activity and Cardiometabolic Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| T2D | Type 2 Diabetes |
| CVD | Cardiovascular Disease |
| AIx | Augmentation Index |
| PWV | Pulse Wave Velocity |
| SEVR | subendocardial viability ratio |
| HbA1c | Hemoglobin A1c |
| BMI | Body Mass Index |
| FIT | Fitbit Intervention group |
| CON | Control group |
| EE | Energy Expenditure |
| GPAQ | Global Physical Activity Questionnaire |
| MET | Metabolic Equivalent of Task |
| SBP | Systolic Blood Pressure |
| DBP | Diastolic Blood Pressure |
| PP | Pulse Pressure |
| MAP | Mean Arterial Pressure |
| WHR | Waist-to Hip Ratio |
Appendix A
| Domain | FIT (n = 5) | CON (n = 3) | ||
|---|---|---|---|---|
| Baseline | Week 4 | Baseline | Week 4 | |
| Work-related Activity | ||||
| Vigorous Intensity (Yes; %) | 0 | 1 (20%) | 0 | 2 (66.7%) |
| Moderate Intensity (Yes; %) | 1 (20%) | 3 (60%) | 1 (33.3%) | 0 |
| MET-min/week | 0 [0–480] | 420 [0–5760] | 0 [0–5760] | 6720 [0–12,960] |
| Transport Activity | ||||
| Walking/Bicycling ≥ 10 min (Yes; %) | 2 (40%) | 4 (80%) | 2 (66.7%) | 2 (66.7%) |
| MET-min/week | 0 [0–140] | 480 [0–1120] | 450 [0–480] | 240 [0–500] |
| Recreational Activity | ||||
| Vigorous Intensity (Yes; %) | 1 (20%) | 0 | 0 | 0 |
| Moderate Intensity (Yes; %) | 3 (60%) | 2 (40%) | 1 (33.3%) | 1 (33.3%) |
| MET-min/week | 480 [0–820] | 0 [0–1440] | 0 [0–660] | 0 [0–600] |
| Total Physical Activity (MET-min/week) | 560 [0–1100] | 1440 [0–6560] | 450 [0–6900] | 6720 [450–14,040] |
| Sedentary Behavior | ||||
| Sitting Time (min/day) | 360 [300–720] | 180 [180–720] | 240 [180–720] | 240 [210–360] |
References
- International Diabetes Federation. Facts & Figures. Available online: https://idf.org/about-diabetes/diabetes-facts-figures/ (accessed on 14 May 2025).
- Centers for Disease Control and Prevention. National Diabetes Statistics Report. Available online: https://www.cdc.gov/diabetes/php/data-research/index.html (accessed on 14 May 2025).
- Raghavan, S.; Vassy, J.L.; Ho, Y.L.; Song, R.J.; Gagnon, D.R.; Cho, K.; Wilson, P.W.F.; Phillips, L.S. Diabetes Mellitus-Related All-Cause and Cardiovascular Mortality in a National Cohort of Adults. J. Am. Heart Assoc. 2019, 8, e011295. [Google Scholar] [CrossRef]
- Hjerkind, K.V.; Stenehjem, J.S.; Nilsen, T.I. Adiposity, physical activity and risk of diabetes mellitus: Prospective data from the population-based HUNT study, Norway. BMJ Open 2017, 7, e013142. [Google Scholar] [CrossRef]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar] [CrossRef]
- Tuomilehto, J.; Lindström, J.; Eriksson, J.G.; Valle, T.T.; Hämäläinen, H.; Ilanne-Parikka, P.; Keinänen-Kiukaanniemi, S.; Laakso, M.; Louheranta, A.; Rastas, M.; et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N. Engl. J. Med. 2001, 344, 1343–1350. [Google Scholar] [CrossRef]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.-P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef] [PubMed]
- Eckert, K. Impact of physical activity and bodyweight on health-related quality of life in people with type 2 diabetes. Diabetes Metab. Syndr. Obes. 2012, 5, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, M.; Gallagher, R.; Neubeck, L.; Bauman, A.; Prebill, G.; Kirkness, A.; Randall, S. Exercise barriers and the relationship to self-efficacy for exercise over 12 months of a lifestyle-change program for people with heart disease and/or diabetes. Eur. J. Cardiovasc. Nurs. 2017, 16, 309–317. [Google Scholar] [CrossRef]
- Schmidt, S.K.; Hemmestad, L.; MacDonald, C.S.; Langberg, H.; Valentiner, L.S. Motivation and Barriers to Maintaining Lifestyle Changes in Patients with Type 2 Diabetes after an Intensive Lifestyle Intervention (The U-TURN Trial): A Longitudinal Qualitative Study. Int. J. Environ. Res. Public Health 2020, 17, 7454. [Google Scholar] [CrossRef]
- Strecher, V.J.; DeVellis, B.M.; Becker, M.H.; Rosenstock, I.M. The role of self-efficacy in achieving health behavior change. Health Educ. Q. 1986, 13, 73–92. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.R.; Li, G.W.; Hu, Y.H.; Yang, W.-Y.; An, Z.-X.; Hu, Z.-X.; Lin, J.; Xiao, J.-Z.; Cao, H.-B.; Liu, P.-A.; et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 1997, 20, 537–544. [Google Scholar] [CrossRef]
- Kim, B.Y.; Lee, J. Smart Devices for Older Adults Managing Chronic Disease: A Scoping Review. JMIR Mhealth Uhealth 2017, 5, e69. [Google Scholar] [CrossRef]
- Tudor-Locke, C.; Aguiar, E.J. Toward comprehensive step-based physical activity guidelines: Are we ready? Kinesiol. Rev. 2019, 8, 25–31. [Google Scholar] [CrossRef]
- Dasgupta, K.; Rosenberg, E.; Joseph, L.; Cooke, A.B.; Trudeau, L.; Bacon, S.L.; Chan, D.; Sherman, M.; Rabasa-Lhoret, R.; Daskalopoulou, S.S.; et al. Physician step prescription and monitoring to improve ARTERial health (SMARTER): A randomized controlled trial in patients with type 2 diabetes and hypertension. Diabetes Obes. Metab. 2017, 19, 695–704. [Google Scholar] [CrossRef]
- Baskerville, R.; Ricci-Cabello, I.; Roberts, N.; Farmer, A. Impact of accelerometer and pedometer use on physical activity and glycaemic control in people with Type 2 diabetes: A systematic review and meta-analysis. Diabet. Med. 2017, 34, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Hodkinson, A.; Kontopantelis, E.; Adeniji, C.; van Marwijk, H.; McMillian, B.; Bower, P.; Panagioti, M. Interventions Using Wearable Physical Activity Trackers Among Adults With Cardiometabolic Conditions: A Systematic Review and Meta-analysis. JAMA Netw. Open 2021, 4, e2116382. [Google Scholar] [CrossRef]
- Gagnon, M.P.; Ouellet, S.; Attisso, E.; Supper, W.; Amil, S.; Rhéaume, C.; Paquette, J.-S.; Chabot, C.; Laferrière, M.-C.; Sasseville, M. Wearable Devices for Supporting Chronic Disease Self-Management: Scoping Review. Interact. J. Med. Res. 2024, 13, e55925. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.; Zhang, N.; Huang, J.; Jiao, X.; Shen, Y. Effectiveness of Wearable Activity Monitors on Metabolic Outcomes in Patients With Type 2 Diabetes: A Systematic Review and Meta-Analysis. Endocr. Pract. 2023, 29, 368–378. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, K.; Xu, Y.; Tao, Y.; Zhang, Q. Effectiveness of Wearable Device-based Intervention on Glycemic Control in Patients with Type 2 Diabetes: A System Review and Meta-Analysis. J. Med. Syst. 2021, 46, 11. [Google Scholar] [CrossRef]
- Kooiman, T.J.M.; de Groot, M.; Hoogenberg, K.; Krijnen, W.P.; van der Schans, C.P.; Kooy, A. Self-tracking of Physical Activity in People With Type 2 Diabetes: A Randomized Controlled Trial. Comput. Inform. Nurs. 2018, 36, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Azar, K.M.; Koliwad, S.; Poon, T.; Xiao, L.; Lv, N.; Griggs, R.; Ma, J. The Electronic CardioMetabolic Program (eCMP) for Patients With Cardiometabolic Risk: A Randomized Controlled Trial. J. Med. Internet Res. 2016, 18, e134. [Google Scholar] [CrossRef]
- American Diabetes Association. Blood Glucose & A1C—Understanding Diabetes Diagnosis. Available online: https://diabetes.org/about-diabetes/diagnosis (accessed on 30 May 2025).
- Waxman, A. WHO global strategy on diet, physical activity and health. Food Nutr. Bull. 2004, 25, 292–302. [Google Scholar] [CrossRef]
- Cleland, C.L.; Hunter, R.F.; Kee, F.; Cupples, M.E.; Sallis, J.F.; Tully, M.A. Validity of the Global Physical Activity Questionnaire (GPAQ) in assessing levels and change in moderate-vigorous physical activity and sedentary behaviour. BMC Public Health 2014, 14, 1255. [Google Scholar] [CrossRef] [PubMed]
- Milton, K.; Bull, F.C.; Bauman, A. Reliability and validity testing of a single-item physical activity measure. Br. J. Sports Med. 2011, 45, 203–208. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Physical Activity Questionnaire (GPAQ)—Analysis Guide; World Health Organization: Geneva, Switzerland, 2021; Available online: https://www.who.int/docs/default-source/ncds/ncd-surveillance/gpaq-analysis-guide.pdf (accessed on 30 May 2025).
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: Abingdon, UK, 1988. [Google Scholar]
- Szeto, K.; Arnold, J.; Singh, B.; Gower, B.; Simpson, C.E.M.; Maher, C. Interventions Using Wearable Activity Trackers to Improve Patient Physical Activity and Other Outcomes in Adults Who Are Hospitalized: A Systematic Review and Meta-analysis. JAMA Netw. Open 2023, 6, e2318478. [Google Scholar] [CrossRef] [PubMed]
- Blood Pressure Lowering Treatment Trialists’ Collaboration. Pharmacological blood pressure lowering for primary and secondary prevention of cardiovascular disease across different levels of blood pressure: An individual participant-level data meta-analysis. Lancet 2021, 397, 1625–1636. [Google Scholar] [CrossRef]
- Franklin, S.S.; Khan, S.A.; Wong, N.D.; Larson, M.G.; Levy, D. Is pulse pressure useful in predicting risk for coronary heart Disease? The Framingham heart study. Circulation 1999, 100, 354–360. [Google Scholar] [CrossRef]
- Safar, M.E. Pulse pressure, arterial stiffness and wave reflections (augmentation index) as cardiovascular risk factors in hypertension. Ther. Adv. Cardiovasc. Dis. 2008, 2, 13–24. [Google Scholar] [CrossRef]
- Ho, L.Y.W.; Kwan, R.Y.C.; Yuen, K.M.; Leung, W.C.; Ni Tam, P.; Tsim, N.M.; Ng, S.S.M.; Heyn, P.C. The effect of aerobic exercises on arterial stiffness in older people: A systematic review and meta-analysis. Gerontologist 2024, 64, gnad123. [Google Scholar] [CrossRef]
- Ambelu, T.; Teferi, G. The impact of exercise modalities on blood glucose, blood pressure and body composition in patients with type 2 diabetes mellitus. BMC Sports Sci. Med. Rehabil. 2023, 15, 153. [Google Scholar] [CrossRef]
- Umpierre, D.; Ribeiro, P.A.; Kramer, C.K.; Leitão, C.B.; Zucatti, A.T.N.; Azevedo, M.J.; Gross, J.L.; Ribeiro, J.P.; Schaan, B.D. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: A systematic review and meta-analysis. JAMA 2011, 305, 1790–1799. [Google Scholar] [CrossRef]
- Boulé, N.G.; Haddad, E.; Kenny, G.P.; Wells, G.A.; Sigal, R.J. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: A meta-analysis of controlled clinical trials. JAMA 2001, 286, 1218–1227. [Google Scholar] [CrossRef]
- Colberg, S.R.; Sigal, R.J.; Fernhall, B.; Regensteiner, J.G.; Blissmer, B.J.; Rubin, R.R.; Chasan-Taber, L.; Albright, A.L.; Braun, B. Exercise and type 2 diabetes: The American College of Sports Medicine and the American Diabetes Association: Joint position statement. Diabetes Care 2010, 33, e147–e167. [Google Scholar] [CrossRef]
- Fiedler, J.; Eckert, T.; Burchartz, A.; Woll, A.; Wunsch, K. Comparison of Self-Reported and Device-Based Measured Physical Activity Using Measures of Stability, Reliability, and Validity in Adults and Children. Sensors 2021, 21, 2672. [Google Scholar] [CrossRef] [PubMed]
- Prince, S.A.; Adamo, K.B.; Hamel, M.E.; Hardt, J.; Connor Gorber, S.; Tremblay, M. A comparison of direct versus self-report measures for assessing physical activity in adults: A systematic review. Int. J. Behav. Nutr. Phys. Act. 2008, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Skender, S.; Ose, J.; Chang-Claude, J.; Paskow, M.; Brühmann, B.; Siegel, E.M.; Steindorf, K.; Ulrich, C.M. Accelerometry and physical activity questionnaires - a systematic review. BMC Public Health 2016, 16, 515. [Google Scholar] [CrossRef] [PubMed]



| Measure | FIT (n = 5) | CON (n = 3) | ||
|---|---|---|---|---|
| Baseline | Week 4 | Baseline | Week 4 | |
| Age (years) | 51 [43–67] | – | 51 [47–59] | – |
| Female (N; %) | 1 (20%) | – | 1 (33.3%) | – |
| Weight (kg) | 72.7 [64.2–78.2] | 71.8 [64.1–79.1] | 79.1 [75.5–124.8] | 78.2 [75.5–124.1] |
| Height (cm) | 175.0 [169.0–175.0] | 175.0 [168.5–175.0] | 175.0 [171.8–178.0] | 175.5 [172.2–178.0] |
| Body Mass Index (BMI) | 23.7 [23.0–27.4] | 23.5 [23.2–27.9] | 27.9 [25.7–40.0] | 27.4 [25.5–39.8] |
| Waist Circumference (cm) | 95.2 [89.0–101.5] | 94.5 [89.0–98.8] | 98.8 [96.6–134.1] | 102.2 [100.1–137.1] |
| Hip Circumference (cm) | 99.1 [93.5–106.0] | 98.8 [94.8–109.2] | 98.8 [96.6–134.1] | 104.0 [102.5–138.0] |
| Waist-to-Hip Ratio | 1.0 [1.0–1.0] | 0.9 [0.9–1.0] | 1.0 [0.9–1.0] | 1.0 [1.0–1.0] |
| Fasting Glucose (mg/dL) | 180.5 [122.5–193.0] | 133.2 [128.4–139.1] | 109.2 [104.0–137.6] | 224.5 [197.5–270.2] |
| Heart Rate (bpm) | 79.0 [73.5–79.0] | 63.5 [59.5–67.4] | 60.0 [59.0–63.5] | 66.0 [61.5–80.0] |
| Systolic Blood Pressure (mmHg) | 139.0 [130.0–142.0] | 117.0 [110.5–123.0] *§ | 110.5 [98.8–116.8] | 125.0 [109.5–130.2] § |
| Diastolic Blood Pressure (mmHg) | 80.0 [79.5–86.0] | 70.0 [63.0–79.5] *§ | 70.0 [66.5–74.8] | 72.0 [68.8–80.0] § |
| Pulse Pressure (mmHg) | 51.0 [44.0–62.0] | 32.0 [31.0–53.0] *§ | 31.0 [27.5–42.0] | 47.5 [38.0–50.2] § |
| Mean Arterial Pressure (mmHg) | 100.7 [100.7–103.5] | 87.7 [81.7–89.8] *§ | 87.7 [79.3–88.8] | 89.7 [82.3–96.7] § |
| Augmentation Index HR75 (%) | 30.0 [25.0–30.0] | 28.0 [24.0–31.9] | 31.5 [28.0–32.2] | 23.0 [21.8–30.8] |
| Pulse Wave Velocity (m/s) | 7.0 [6.9–9.7] | 6.8 [6.7–7.6] | 7.5 [7.0–7.9] | 7.8 [7.0–8.6] |
| Subendocardial Viability Ratio (%) | 118.0 [105.0–124.0] | 153.2 [144.4–160.9] | 160.0 [149.0–161.8] | 144.0 [124.2–149.0] |
| Step Count (steps/day) | 5296.0 [5267.0–5573.0] | 8558.2 [7160.6–9162.2] | 5691.1 [4862.6–6203.6] | 5961.7 [3252.2–6257.2] |
| Walking Distance (km/day) | 3.8 [3.5–4.0] | 6.1 [4.7–6.5] *§ | 4.1 [3.6–4.4] | 3.8 [2.1–4.1] § |
| Energy Expenditure (kcal/day) | 1704.4 [1682.6–1726.0] | 2385.8 [2291.6–2700.0] *§ | 1737.6 [1711.9–2334.2] | 1726.8 [1715.9–2181.7] § |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, P.-T.; Baltich, A.A.; Chu, I.-H.; Chui, K.K. Wearable Activity Trackers to Improve Physical Activity and Cardiovascular Risk in Type 2 Diabetes: A Randomized Pilot Study. Diabetology 2025, 6, 97. https://doi.org/10.3390/diabetology6090097
Wu P-T, Baltich AA, Chu I-H, Chui KK. Wearable Activity Trackers to Improve Physical Activity and Cardiovascular Risk in Type 2 Diabetes: A Randomized Pilot Study. Diabetology. 2025; 6(9):97. https://doi.org/10.3390/diabetology6090097
Chicago/Turabian StyleWu, Pei-Tzu, Ashlee A. Baltich, I-Hua Chu, and Kevin K. Chui. 2025. "Wearable Activity Trackers to Improve Physical Activity and Cardiovascular Risk in Type 2 Diabetes: A Randomized Pilot Study" Diabetology 6, no. 9: 97. https://doi.org/10.3390/diabetology6090097
APA StyleWu, P.-T., Baltich, A. A., Chu, I.-H., & Chui, K. K. (2025). Wearable Activity Trackers to Improve Physical Activity and Cardiovascular Risk in Type 2 Diabetes: A Randomized Pilot Study. Diabetology, 6(9), 97. https://doi.org/10.3390/diabetology6090097






