Urinary Bisphenol Mixtures at Population-Exposure Levels Are Associated with Diabetes Prevalence: Evidence from Advanced Mixture Modeling
Abstract
1. Introduction
2. Materials and Methods
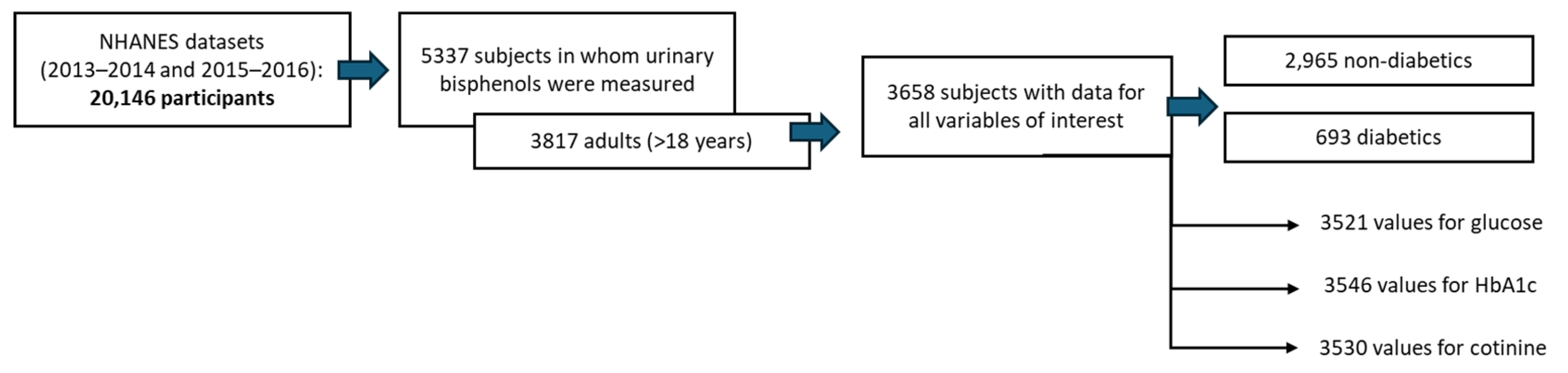
2.1. Data Extraction from the NHANES Cohort
2.2. Combined Urinary Bisphenol Exposure Study
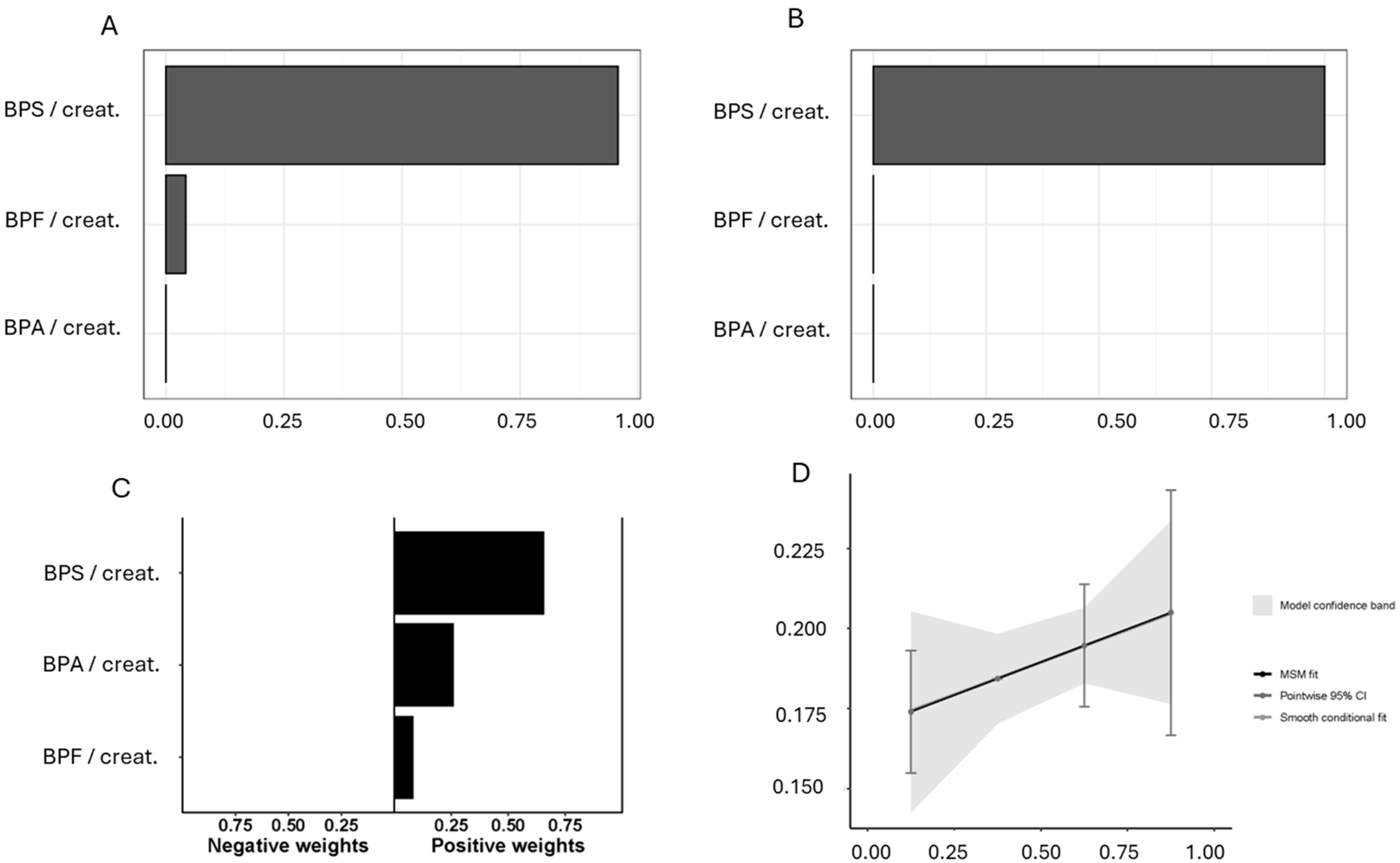
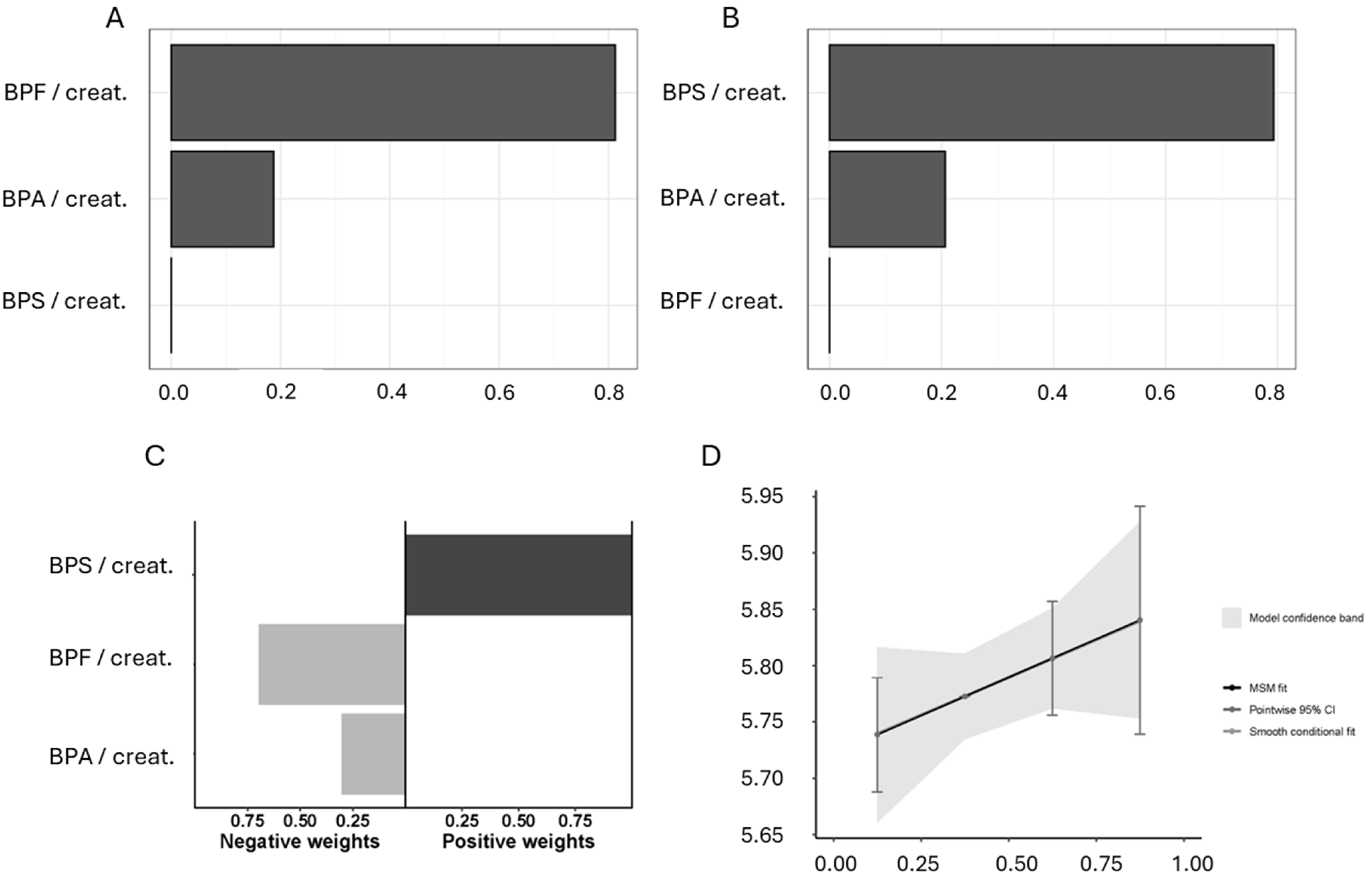
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Porta, R. Anthropocene, the plastic age and future perspectives. FEBS Open Bio 2021, 11, 948–953. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Hauser, R.; Marcus, M.; Olea, N.; Welshons, W.V. Human exposure to bisphenol A (BPA). Reprod. Toxicol. 2007, 24, 139–177. [Google Scholar] [CrossRef]
- Vogel, S.A. The politics of plastics: The making and unmaking of bisphenol a ‘safety’. Am. J. Public Health 2009, 99 (Suppl. 3), 559–566. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Chahoud, I.; Heindel, J.J.; Padmanabhan, V.; Paumgartten, F.J.; Schoenfelder, G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ. Health Perspect. 2010, 118, 1055–1070. [Google Scholar] [CrossRef]
- Hermabessiere, L.; Dehaut, A.; Paul-Pont, I.; Lacroix, C.; Jezequel, R.; Soudant, P.; Duflos, G. Occurrence and effects of plastic additives on marine environments and organisms: A review. Chemosphere 2017, 182, 781–793. [Google Scholar] [CrossRef]
- Lee, T.; Jung, S.; Baek, K.; Tsang, Y.F.; Lin, K.A.; Jeon, Y.J.; Kwon, E.E. Functional use of CO2 to mitigate the formation of bisphenol A in catalytic pyrolysis of polycarbonate. J. Hazard. Mater. 2022, 423, 126992. [Google Scholar] [CrossRef]
- Walker, T.R. (Micro)plastics and the UN sustainable development goals. Curr. Opin. Green Sustain. Chem. 2021, 30, 100497. [Google Scholar] [CrossRef]
- De Kermoysan, G.; Joachim, S.; Baudoin, P.; Lonjaret, M.; Tebby, C.; Lesaulnier, F.; Lestremau, F.; Chatellier, C.; Akrour, Z.; Pheron, E.; et al. Effects of bisphenol A on different trophic levels in a lotic experimental ecosystem. Aquat. Toxicol. 2013, 144–145, 186–198. [Google Scholar] [CrossRef]
- Substance Information—ECHA. Available online: https://echa.europa.eu/es/substance-information/-/substanceinfo/100.001.133 (accessed on 23 March 2024).
- Moreno-Gómez-Toledano, R. Exploring the Bisphenol A Paradigm: Historical Evolution, Environmental Presence, Pharmacokinetics, and Societal Impact. In Bisphenols-New Environmental, Pathophysiological and Social Perspectives; IntechOpen: London, UK, 2025. [Google Scholar] [CrossRef]
- Wu, L.H.; Zhang, X.M.; Wang, F.; Gao, C.J.; Chen, D.; Palumbo, J.R.; Guo, Y.; Zeng, E.Y. Occurrence of bisphenol S in the environment and implications for human exposure: A short review. Sci. Total Environ. 2018, 615, 87–98. [Google Scholar] [CrossRef]
- Vasiljevic, T.; Harner, T. Bisphenol A and its analogues in outdoor and indoor air: Properties, sources and global levels. Sci. Total Environ. 2021, 789, 148013. [Google Scholar] [CrossRef]
- Mishra, A.; Goel, D.; Shankar, S. Bisphenol A contamination in aquatic environments: A review of sources, environmental concerns, and microbial remediation. Environ. Monit. Assess. 2023, 195, 1352. [Google Scholar] [CrossRef]
- Dueñas-Moreno, J.; Mora, A.; Cervantes-Avilés, P.; Mahlknecht, J. Groundwater contamination pathways of phthalates and bisphenol A: Origin, characteristics, transport, and fate—A review. Environ. Int. 2022, 170, 107550. [Google Scholar] [CrossRef]
- Gayrard, V.; Lacroix, M.Z.; Grandin, F.C.; Collet, S.H.; Mila, H.; Viguié, C.; Gély, C.A.; Rabozzi, B.; Bouchard, M.; Léandri, R. Oral Systemic Bioavailability of Bisphenol A and Bisphenol S in Pigs. Environ. Health Perspect. 2019, 127, 77005. [Google Scholar] [CrossRef]
- Danzl, E.; Sei, K.; Soda, S.; Ike, M.; Fujita, M. Biodegradation of bisphenol A, bisphenol F and bisphenol S in seawater. Int. J. Environ. Res. Public Health 2009, 6, 1472–1484. [Google Scholar] [CrossRef]
- Fan, W. Epidemiology in diabetes mellitus and cardiovascular disease. Cardiovasc. Endocrinol. 2017, 6, 8. [Google Scholar] [CrossRef]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef]
- Glovaci, D.; Fan, W.; Wong, N.D. Epidemiology of Diabetes Mellitus and Cardiovascular Disease. Curr. Cardiol. Rep. 2019, 21, 21. [Google Scholar] [CrossRef]
- Yang, B.Y.; Fan, S.; Thiering, E.; Seissler, J.; Nowak, D.; Dong, G.H.; Heinrich, J. Ambient air pollution and diabetes: A systematic review and meta-analysis. Environ. Res. 2020, 180, 108817. [Google Scholar] [CrossRef]
- Rochester, J.R. Bisphenol A and human health: A review of the literature. Reprod. Toxicol. 2013, 42, 132–155. [Google Scholar] [CrossRef]
- La Merrill, M.A.; Vandenberg, L.N.; Smith, M.T.; Goodson, W.; Browne, P.; Patisaul, H.B.; Guyton, K.Z.; Kortenkamp, A.; Cogliano, V.J.; Woodruff, T.J.; et al. Consensus on the key characteristics of endocrine-disrupting chemicals as a basis for hazard identification. Nat. Rev. Endocrinol. 2020, 16, 45–57. [Google Scholar] [CrossRef]
- Farrugia, F.; Aquilina, A.; Vassallo, J.; Pace, N.P. Bisphenol A and Type 2 Diabetes Mellitus: A Review of Epidemiologic, Functional, and Early Life Factors. Int. J. Environ. Res. Public Health 2021, 18, 716. [Google Scholar] [CrossRef]
- Siracusa, J.S.; Yin, L.; Measel, E.; Liang, S.; Yu, X. Effects of bisphenol A and its analogs on reproductive health: A mini review. Reprod. Toxicol. 2018, 79, 96–123. [Google Scholar] [CrossRef]
- Moreno-Gómez-Toledano, R.; Delgado-Marín, M.; Cook-Calvete, A.; González-Cucharero, C.; Alcharani, N.; Jiménez-Guirado, B.; Hernandez, I.; Ramirez-Carracedo, R.; Tesoro, L.; Botana, L. New environmental factors related to diabetes risk in humans: Emerging bisphenols used in synthesis of plastics. World J. Diabetes 2023, 14, 1301–1313. [Google Scholar] [CrossRef]
- Lehmler, H.-J.; Liu, B.Y.; Gadogbe, M.; Bao, W. Exposure to Bisphenol A, Bisphenol F, and Bisphenol S in U.S. Adults and Children: The National Health and Nutrition Examination Survey 2013–2014. ACS Omega 2018, 3, 6523–6532. [Google Scholar] [CrossRef]
- Zhang, W.; Xia, W.; Liu, W.; Li, X.; Hu, J.; Zhang, B.; Xu, S.; Zhou, Y.; Li, J.; Cai, Z.; et al. Exposure to bisphenol A substitutes and gestational diabetes mellitus: A prospective cohort study in China. Front. Endocrinol. 2019, 10, 262. [Google Scholar] [CrossRef]
- Tang, P.; Liang, J.; Liao, Q.; Huang, H.; Guo, X.; Lin, M.; Liu, B.; Wei, B.; Zeng, X.; Liu, S.; et al. Associations of bisphenol exposure with the risk of gestational diabetes mellitus: A nested case–control study in Guangxi, China. Environ. Sci. Pollut. Res. 2023, 30, 25170–25180. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). National Health and Nutrition Examination Survey Data. National Center for Health Statistics (NCHS). Available online: https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?BeginYear=2013 (accessed on 15 January 2022).
- Moreno-Gomez-Toledano, R.; Velez-Velez, E.; Arenas, I.M.; Saura, M.; Bosch, R.J. Association between urinary concentrations of bisphenol A substitutes and diabetes in adults. World J. Diabetes 2022, 13, 521–531. [Google Scholar] [CrossRef]
- Li, M.C.; Mínguez-Alarcón, L.; Bellavia, A.; Williams, P.L.; James-Todd, T.; Hauser, R.; Chavarro, J.E.; Chiu, Y.H. Serum beta-carotene modifies the association between phthalate mixtures and insulin resistance: The National Health and Nutrition Examination Survey 2003–2006. Environ. Res. 2019, 178, 108729. [Google Scholar] [CrossRef]
- 2013–2014 Laboratory Data—Continuous NHANES. Available online: https://wwwn.cdc.gov/nchs/nhanes/search/datapage.aspx?Component=Laboratory&Cycle=2013-2014 (accessed on 11 April 2023).
- Pirkle, J.L. Laboratory Procedure Manual Benzophenone-3, Bisphenol A, Bisphenol F, Bisphenol S, 2,4-Dichlorophenol, 2,5-Dichlorophenol, Methyl-, Ethyl-, Propyl-, and Butyl Parabens, Triclosan, and Triclocarban on Line SPE-HPLC-Isotope Dilution-MS/MS. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/public/2013/labmethods/EPHPP_H_MET.pdf (accessed on 11 April 2023).
- Trasande, L.; Spanier, A.J.; Sathyanarayana, S.; Attina, T.M.; Blustein, J. Urinary Phthalates and Increased Insulin Resistance in Adolescents. Pediatrics 2013, 132, E646–E655. [Google Scholar] [CrossRef]
- Stahlhut, R.W.; van Wijngaarden, E.; Dye, T.D.; Cook, S.; Swan, S.H. Concentrations of urinary phthalate metabolites are associated with increased waist circumference and insulin resistance in adult U.S. males. Environ. Health Perspect. 2007, 115, 876–882. [Google Scholar] [CrossRef]
- Moon, S.; Yu, S.H.; Lee, C.B.; Park, Y.J.; Yoo, H.J.; Kim, D.S. Effects of bisphenol A on cardiovascular disease: An epidemiological study using National Health and Nutrition Examination Survey 2003–2016 and meta-analysis. Sci. Total Environ. 2021, 763, 142941. [Google Scholar] [CrossRef]
- Pirkle, J.L. Exposure of the US Population to Environmental Tobacco Smoke. JAMA 1996, 275, 1233. [Google Scholar] [CrossRef]
- Renzetti, S.; Curtin, P.; Just, A.C.; Bello, G.; Gennings, C. _gWQS: Generalized Weighted Quantile Sum Regression_. R Package Version 3.0.4. Available online: https://CRAN.R-project.org/package=gWQS (accessed on 1 November 2024).
- Carrico, C.; Gennings, C.; Wheeler, D.C.; Factor-Litvak, P. Characterization of Weighted Quantile Sum Regression for Highly Correlated Data in a Risk Analysis Setting. J. Agric. Biol. Environ. Stat. 2015, 20, 100–120. [Google Scholar] [CrossRef]
- Czarnota, J.; Gennings, C.; Wheeler, D.C. Assessment of weighted quantile sum regression for modeling chemical mixtures and cancer risk. Cancer Inform. 2015, 14, 159–171. [Google Scholar] [CrossRef]
- Colicino, E.; Pedretti, N.F.; Busgang, S.A.; Gennings, C. Per- and poly-fluoroalkyl substances and bone mineral density: Results from the Bayesian weighted quantile sum regression. Environ. Epidemiol. 2020, 4, e092. [Google Scholar] [CrossRef]
- Keil, A.; _qgcomp: Quantile G-Computation_. R Package Version 2.10.1. Available online: https://CRAN.R-project.org/package=qgcomp (accessed on 1 November 2024).
- Rochester, J.R.; Bolden, A.L. Bisphenol S and F: A systematic review and comparison of the hormonal activity of bisphenol a substitutes. Environ. Health Perspect. 2015, 123, 643–650. [Google Scholar] [CrossRef]
- Ramskov Tetzlaff, C.N.; Svingen, T.; Vinggaard, A.M.; Rosenmai, A.K.; Taxvig, C. Bisphenols B, E, F, and S and 4-cumylphenol induce lipid accumulation in mouse adipocytes similarly to bisphenol A. Environ. Toxicol. 2020, 35, 543–552. [Google Scholar] [CrossRef]
- Sidorkiewicz, I.; Czerniecki, J.; Jarzabek, K.; Zbucka-Krętowska, M.; Wołczyński, S. Cellular, transcriptomic and methylome effects of individual and combined exposure to BPA, BPF, BPS on mouse spermatocyte GC-2 cell line. Toxicol. Appl. Pharmacol. 2018, 359, 1–11. [Google Scholar] [CrossRef]
- Manzoor, M.F.; Tariq, T.; Fatima, B.; Sahar, A.; Tariq, F.; Munir, S.; Khan, S.; Nawaz Ranjha, M.M.A.; Sameen, A.; Zeng, X.A.; et al. An insight into bisphenol A, food exposure and its adverse effects on health: A review. Front. Nutr. 2022, 9, 1047827. [Google Scholar] [CrossRef]
- Nelson, J.W.; Scammell, M.K.; Hatch, E.E.; Webster, T.F. Social disparities in exposures to bisphenol A and polyfluoroalkyl chemicals: A cross-sectional study within NHANES 2003-2006. Environ. Health 2012, 11, 10. [Google Scholar] [CrossRef]
- Sargis, R.M.; Simmons, R.A. Environmental neglect: Endocrine disruptors as underappreciated but potentially modifiable diabetes risk factors. Diabetologia 2019, 62, 1811. [Google Scholar] [CrossRef]
- Hartle, J.C.; Navas-Acien, A.; Lawrence, R.S. The consumption of canned food and beverages and urinary Bisphenol A concentrations in NHANES 2003–2008. Environ. Res. 2016, 150, 375–382. [Google Scholar] [CrossRef]
- Lim, D.S.; Kwack, S.J.; Kim, K.B.; Kim, H.S.; Lee, B.M. Potential Risk of Bisphenol a Migration From Polycarbonate Containers After Heating, Boiling, and Microwaving. J. Toxicol. Environ. Health A 2009, 72, 1285–1291. [Google Scholar] [CrossRef]
- Pan, Y.; Wu, M.; Shi, M.; Shi, P.; Zhao, N.; Zhu, Y.; Karimi-Maleh, H.; Ye, C.; Lin, C.T.; Fu, L. An Overview to Molecularly Imprinted Electrochemical Sensors for the Detection of Bisphenol A. Sensors 2023, 23, 8656. [Google Scholar] [CrossRef]
- Shankar, A.; Teppala, S. Urinary bisphenol A and hypertension in a multiethnic sample of US adults. J. Environ. Public Health 2012, 2012, 481641. [Google Scholar] [CrossRef]
- Zhu, Y.; Hedderson, M.M.; Calafat, A.M.; Alexeeff, S.E.; Feng, J.; Quesenberry, C.P.; Ferrara, A. Urinary Phenols in Early to Midpregnancy and Risk of Gestational Diabetes Mellitus: A Longitudinal Study in a Multiracial Cohort. Diabetes 2022, 71, 2539–2551. [Google Scholar] [CrossRef]
- Mentor, A.; Wann, M.; Brunström, B.; Jönsson, M.; Mattsson, A. Bisphenol AF and bisphenol F induce similar feminizing effects in chicken embryo testis as bisphenol A. Toxicol. Sci. 2020, 178, 239–250. [Google Scholar] [CrossRef]
- Alharbi, H.F.; Algonaiman, R.; Alduwayghiri, R.; Aljutaily, T.; Algheshairy, R.M.; Almutairi, A.S.; Alharbi, R.M.; Alfurayh, L.A.; Alshahwan, A.A.; Alsadun, A.F.; et al. Exposure to Bisphenol A Substitutes, Bisphenol S and Bisphenol F, and Its Association with Developing Obesity and Diabetes Mellitus: A Narrative Review. Int. J. Environ. Res. Public Health 2022, 19, 15918. [Google Scholar] [CrossRef]
- Kim, J.I.; Lee, Y.A.; Shin, C.H.; Hong, Y.C.; Kim, B.N.; Lim, Y.H. Association of bisphenol A, bisphenol F, and bisphenol S with ADHD symptoms in children. Environ. Int. 2022, 161, 107093. [Google Scholar] [CrossRef]
- Marroqui, L.; Martinez-Pinna, J.; Castellano-Muñoz, M.; Dos Santos, R.S.; Medina-Gali, R.M.; Soriano, S.; Quesada, I.; Gustafsson, J.A.; Encinar, J.A.; Nadal, A. Bisphenol-S and Bisphenol-F alter mouse pancreatic β-cell ion channel expression and activity and insulin release through an estrogen receptor ERβ mediated pathway. Chemosphere 2021, 265, 129051. [Google Scholar] [CrossRef]
- Ahmed, F.; Pereira, M.J.; Aguer, C. Bisphenols and the Development of Type 2 Diabetes: The Role of the Skeletal Muscle and Adipose Tissue. Environments 2021, 8, 35. [Google Scholar] [CrossRef]
- Zhao, F.; Jiang, G.; Wei, P.; Wang, H.; Ru, S. Bisphenol S exposure impairs glucose homeostasis in male zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2018, 147, 794–802. [Google Scholar] [CrossRef]
- Duan, Y.; Sun, H.; Yao, Y.; Han, L.; Chen, L. Perturbation of serum metabolome in relation to type 2 diabetes mellitus and urinary levels of phthalate metabolites and bisphenols. Environ. Int. 2021, 155, 106609. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, S.; Song, N.; Guo, R.; Chen, M.; Mai, D.; Yan, Z.; Han, Z.; Chen, J. Occurrence, distribution and sources of bisphenol analogues in a shallow Chinese freshwater lake (Taihu Lake): Implications for ecological and human health risk. Sci. Total Environ. 2017, 599–600, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Lin, C.; Wang, A.; He, M.; Liu, X.; Ouyang, W. Socioeconomic and seasonal effects on spatiotemporal trends in estrogen occurrence and ecological risk within a river across low-urbanized and high-husbandry landscapes. Environ. Int. 2023, 180, 108246. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, J.; Han, L.; Ren, J.; Jing, C.; Lu, G.; Yang, X. Medium distribution, source characteristics and ecological risk of bisphenol compounds in agricultural environment. Emerg. Contam. 2024, 10, 100292. [Google Scholar] [CrossRef]
- Palsania, P.; Singhal, K.; Dar, M.A.; Kaushik, G. Food grade plastics and Bisphenol A: Associated risks, toxicity, and bioremediation approaches. J. Hazard. Mater. 2024, 466, 133474. [Google Scholar] [CrossRef]
- Uthra, K.T.; Chitra, V.; Damodharan, N.; Devadoss, A.; Kuehnel, M.; Exposito, A.J.; Nagarajan, S.; Pitchaimuthu, S.; Pazhani, G.P. Microplastic emerging pollutants—Impact on microbiological diversity, diarrhea, antibiotic resistance, and bioremediation. Environ. Sci. Adv. 2023, 2, 1469–1487. [Google Scholar] [CrossRef]
- Moreno-Gómez-Toledano, R.; Jabal-Uriel, C. The “Plastic Age”: From Endocrine Disruptors to Microplastics—An Emerging Threat to Pollinators. In Environmental Health Literacy Update—New Evidence, Methodologies and Perspectives; IntechOpen: London, UK, 2024. [Google Scholar] [CrossRef]

| Variable | Healthy (n = 2965) | Diabetes (n = 693) | p-Value |
|---|---|---|---|
| Gender, men (%) | 1389 (46.85) | 343 (49.50) | 0.112 |
| Age | 41.07 (40.43–41.71) | 57.08 (55.88–58.31) | 0.000 |
| BMI (kg/m2) | 27.8 (27.58–28.03) | 31.2 (30.69–31.72) | 0.000 |
| Mexican American | 434 (14.64) | 130 (18.76) | 0.000 |
| Other Hispanic | 317 (10.69) | 88 (12.70) | |
| Non-Hispanic White | 1125 (37.94) | 195 (28.14) | |
| Non-Hispanic Black | 640 (21.59) | 174 (25.11) | |
| Other Race—Including Multi-Racial | 449 (15.14) | 106 (15.30) | |
| Poverty-Income Ratio $ | 1.79 (0.83–3.81) | 1.53 (0.78–3.27) | 0.013 |
| Hypertension | 1043 (35.18) | 472 (68.11) | 0.000 |
| Dyslipidemia | 993 (33.19) | 397 (57.29) | 0.000 |
| Smoking | 1266 (42.70) | 337 (48.63) | 0.003 |
| ΣBPs/Creat (µg/g) | 2.87 (2.78–2.97) | 3.2 (2.98–3.43) | 0.017 |
| ΣBP/Mol (nM) | 2.13 (2.03–2.23) | 2.21 (2.02–2.43) | 0.587 |
| BPA/Creat (µg/g) | 1.16 (1.12–1.19) | 1.2 (1.12–1.28) | 0.216 |
| BPS/Creat (µg/g) | 0.50 (0.48–0.53) | 0.57 (0.52–0.62) | 0.018 |
| BPF/Creat (µg/g) | 0.41 (0.39–0.43) | 0.44 (0.40–0.50) | 0.522 |
| Variable | Q1 (n = 914) | Q2 (n = 915) | Q3 (n = 915) | Q4 (n = 914) | p-Value |
|---|---|---|---|---|---|
| Diabetes (%) | 157 (17.2) | 169 (18.5) | 175 (19.1) | 192 (21.0) | 0.208 |
| Gender, men (%) | 533 (58.3) | 420 (45.9) | 386 (42.2) | 393 (43.0) | 0.000 |
| Age | 41.6 (40.5–42.8) | 44.6 (43.4–45.8) ** | 44.1 (42.8–45.3) ** | 44.61 (43.4–45.8) ** | |
| BMI (kg/m2) | 28.3(27.9–28.7) | 28.2 (27.7–28.6) | 28.5 (28.1–28.9) | 28.7 (28.2–29.1) | |
| Mexican American | 139 (15.2) | 150 (16.4) | 144 (15.7) | 131 (14.3) | 0.000 |
| Other Hispanic | 86 (9.4) | 102 (11.1) | 110 (12.0) | 107 (11.7) | |
| Non-Hispanic White | 307 (33.6) | 325 (35.5) | 344 (37.6) | 344 (37.6) | |
| Non-Hispanic Black | 192 (21.0) | 204 (22.3) | 199 (21.7) | 219 (24.0) | |
| Other Race—Including Multi-Racial | 190 (20.8) | 134 (14.6) | 118 (12.9) | 113 (12.4) | |
| Poverty-Income Ratio $ | 2 (0.9–4.1) | 1.8 (0.8–3.6) | 1.7 (0.8–3.6) | 1.6 (0.8–3.4) ** | |
| Hypertension | 338 (37.0) | 396 (43.3) | 378 (41.3) | 403 (44.1) | 0.010 |
| Dyslipidemia | 327 (35.8) | 352 (38.5) | 341 (37.3) | 370 (40.5) | 0.204 |
| Smoking | 360 (239.4) | 370 (40.4) | 419 (45.8) | 454 (49.7) | 0.000 |
| Variable | Q1 (n = 916) | Q2 (n = 915) | Q3 (n = 912) | Q4 (n = 915) | p-Value |
|---|---|---|---|---|---|
| Diabetes (%) | 158 (17.2) | 195 (21.3) | 174 (19.1) | 166 (18.1) | 0.142 |
| Gender, men (%) | 416 (45.4) | 444 (48.5) | 450 (49.3) | 422 (46.1) | 0.274 |
| Age | 45.4 (44.2–46.7) | 44.8 (43.6–46) | 42.4 (41.2–43.6) ** | 42.4 (41.2–43.6) *** | |
| BMI (kg/m2) | 27.1 (26.7–27.5) | 28.2 (27.8–28.6) **** | 29 (28.6–29.5) **** | 29.3 (28.9–29.8) **** | |
| Mexican American | 122 (13.3) | 131 (14.3) | 159 (17.4) | 152 (16.6) | 0.000 |
| Other Hispanic | 69 (7.5) | 110 (12.0) | 119 (13.0) | 107 (11.7) | |
| Non-Hispanic White | 424 (46.3) | 342 (37.4) | 294 (32.2) | 260 (28.4) | |
| Non-Hispanic Black | 131 (14.3) | 183 (20.0) | 213 (23.4) | 287 (31.4) | |
| Other Race—Including Multi-Racial | 170 (18.6) | 149 (16.3) | 127 (13.9) | 109 (11.9) | |
| Poverty-Income Ratio $ | 2.4 (1–4.5) | 1.8 (0.9–3.5) **** | 1.5 (0.7–3.2) **** | 1.5 (0.7–3.3) **** | |
| Hypertension | 364 (39.7) | 395 (43.2) | 362 (39.7) | 394 (43.1) | 0.225 |
| Dyslipidemia | 353 (38.2) | 366 (40.0) | 347 (38.0) | 324 (35.4) | 0.234 |
| Smoking | 372 (40.6) | 417 (45.6) | 409 (44.8) | 405 (44.3) | 0.142 |
| ΣBPs/Creat | ΣBPs/Mol | Glucose | HbA1c | Cholesterol | Cotinine | |
|---|---|---|---|---|---|---|
| ΣBPs/creat | 1 | ** | - | * | - | ** |
| ΣBPs/Mol | 0.391 ** | 1 | - | * | - | ** |
| Glucose | 0.033 | 0.031 | 1 | ** | - | * |
| HbA1c | 0.035 * | 0.36 * | 0.757 ** | 1 | - | * |
| Cholesterol | 0.009 | −0.017 | 0.007 | 0.029 | 1 | - |
| Cotinine | 0.085 ** | 0.078 ** | −0.040 * | −0.033 * | −0.015 | 1 |
| BP Mixture | Covariates | OR (95% CI) | p-Value |
|---|---|---|---|
| ΣBPs/creat | 1 | 1.132 (1.038–1.235) | 0.005 |
| 2 | 1.102 (1.003–1.211) | 0.043 | |
| 3 | 1.103 (1.002–1.214) | 0.045 | |
| ΣBPs/Mol | 1 | 1.024 (0.960–1.092) | 0.468 |
| 2 | 1.024 (0.955–1.097) | 0.509 | |
| 3 | 0.989 (0.920–1.064) | 0.773 |
| Q1 | Q2 | Q3 | Q4 | |
|---|---|---|---|---|
| Variable | Ref. | OR (95% CI) | OR (95%CI) | OR (95%CI) |
| 1 Diabetes (1) | Ref. | 1.09 (0.86–1.39) | 1.14 (0.90–1.45) | 1.28 (1.01–1.62) * |
| 1 Diabetes (2) | Ref. | 1.01 (0.78–1.30) | 1.05 (0.81–1.36) | 1.16 (0.90–1.50) |
| 1 Diabetes (3) | Ref. | 1.03 (0.80–1.34) | 1.10 (0.85–1.43) | 1.21 (0.93–1.56) |
| 2 Diabetes (1) | Ref. | 1.30 (1.03–1.64) * | 1.13 (0.89–1.44) | 1.06 (0.84–1.35) |
| 2 Diabetes (2) | Ref. | 1.27 (0.99–1.62) | 1.12 (0.87–1.45) | 1.04 (0.80–1.35) |
| 2 Diabetes (3) | Ref. | 1.14 (0.89–1.48) | 0.98 (0.76–1.28) | 0.89 (0.68–1.17) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grande-Alonso, M.; Jabal-Uriel, C.; Aguado-Henche, S.; Flores-Sáenz, M.; Méndez-Mesón, I.; Rodríguez Slocker, A.; López González, L.; Ramírez-Carracedo, R.; Sebastián-Martín, A.; Moreno-Gómez-Toledano, R. Urinary Bisphenol Mixtures at Population-Exposure Levels Are Associated with Diabetes Prevalence: Evidence from Advanced Mixture Modeling. Diabetology 2025, 6, 91. https://doi.org/10.3390/diabetology6090091
Grande-Alonso M, Jabal-Uriel C, Aguado-Henche S, Flores-Sáenz M, Méndez-Mesón I, Rodríguez Slocker A, López González L, Ramírez-Carracedo R, Sebastián-Martín A, Moreno-Gómez-Toledano R. Urinary Bisphenol Mixtures at Population-Exposure Levels Are Associated with Diabetes Prevalence: Evidence from Advanced Mixture Modeling. Diabetology. 2025; 6(9):91. https://doi.org/10.3390/diabetology6090091
Chicago/Turabian StyleGrande-Alonso, Mónica, Clara Jabal-Uriel, Soledad Aguado-Henche, Manuel Flores-Sáenz, Irene Méndez-Mesón, Ana Rodríguez Slocker, Laura López González, Rafael Ramírez-Carracedo, Alba Sebastián-Martín, and Rafael Moreno-Gómez-Toledano. 2025. "Urinary Bisphenol Mixtures at Population-Exposure Levels Are Associated with Diabetes Prevalence: Evidence from Advanced Mixture Modeling" Diabetology 6, no. 9: 91. https://doi.org/10.3390/diabetology6090091
APA StyleGrande-Alonso, M., Jabal-Uriel, C., Aguado-Henche, S., Flores-Sáenz, M., Méndez-Mesón, I., Rodríguez Slocker, A., López González, L., Ramírez-Carracedo, R., Sebastián-Martín, A., & Moreno-Gómez-Toledano, R. (2025). Urinary Bisphenol Mixtures at Population-Exposure Levels Are Associated with Diabetes Prevalence: Evidence from Advanced Mixture Modeling. Diabetology, 6(9), 91. https://doi.org/10.3390/diabetology6090091










