Abstract
Background/Objectives: Obesity-related insulin resistance leads to the development of type 2 diabetes mellitus (T2DM); however, the pathophysiology of these metabolic alterations remains incompletely understood. This study aimed to characterize the lipidomic alterations during obesity–insulin resistance–T2DM progression in a high-sugar diet (HSD) model. Methods: Fruit flies were fed either a standard diet (SD) or an HSD and monitored across a 14-day period. Metabolic phenotyping and lipidomic profiling were conducted during the experiment. Results: After two days of HSD, fruit flies exhibited an obesity phenotype (50% increase in triglyceride content) and insulin resistance (hyperglycemia and insulin overexpression), progressing to an early T2DM-like state (increased triglycerides, hyperglycemia, and normal insulin expression) from days 4 to 6, and finally to a late T2DM-like phenotype (increased triglycerides, hyperglycemia, and insulin down-regulation) from days 8 to 14. Multivariate analyses indicated an altered lipidome profile in HSD-fed fruit flies from day 2 until the end of the experiment. Fatty acids and phosphatidylethanolamines (PEs) containing 16:0, 16:1, and 18:1 acyl chains were significantly altered during the development of obesity-related insulin resistance and early T2DM-like state (days 2 to 6); whereas palmitic acid and oleic acid-LysoPE alterations were associated with the onset and progression of obesity-related T2DM-like state (days 4 to 14). Conclusions: The progression from obesity-related insulin resistance to a T2DM-like state in an HSD-fed D. melanogaster model is accompanied by distinct lipidomic signatures involving 16- and 18-carbon fatty acid derivatives. These findings provide insight into potential biomarkers and mechanistic pathways in the early pathogenesis of T2DM.
1. Introduction
Type 2 diabetes mellitus (T2DM) is a disease characterized by a state of hyperglycemia due to the development of insulin resistance. According to the International Diabetes Federation (IDF), 537 million adults worldwide (20–79 years old) were living with DM in 2021, of which 90–95% had T2DM. DM is associated with chronic metabolic complications resulting from persistent hyperglycemia, like retinopathy, neuropathy, nephropathy, cardiovascular, and cerebrovascular diseases, all leading to premature deaths. A total of 6.7 million deaths associated with DM were reported in 2021 [1].
There is growing global interest in research leading to the prevention, control, and treatment of DM and its complications. Several risk factors for T2DM have been identified, including obesity-related insulin resistance, with the latter mainly being associated with the chronic consumption of hypercaloric diets [2]. Increased adipose tissue promotes macrophage infiltration, leading to local and subsequently systemic low-grade inflammation. It is well-established that several pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6), interfere with insulin signaling in insulin-sensitive tissues, decreasing glucose uptake in adipocytes and myocytes. A similar mechanism has been widely associated with obesity-induced elevation of circulating free fatty acids, which contributes further to insulin resistance [3]. Moreover, an altered profile of fatty acids and other complex lipids promotes a lipotoxic environment, exacerbating the insulin resistance state [4].
Despite extensive research, the molecular mechanisms underlying T2DM onset and progression remain incompletely understood. Therefore, extensive efforts have been directed to undercut current knowledge gaps regarding the mechanisms associated with the onset and development of T2DM. According to Vanamala et al. [5], there is an increasing trend toward the use of omics sciences—mainly proteomics, metabolomics, and lipidomics—as analytical tools in T2DM research, providing new insights into the disease pathophysiology.
Palau-Rodriguez et al. [6] recently demonstrated that 18 phospholipid species are altered in adults with obesity and insulin resistance compared to those having insulin sensitivity. Notably, adults with the insulin-resistant obesity phenotype showed lower levels of phospholipids containing C18-fatty acyl groups, including phosphatidylethanolamines (PEs), phosphatidylinositols (PI), phosphatidylserines (PS), and phosphatidylcholines (PCs), which act as lipid mediators in cell signaling. Similar results were reported by Al-Sulaiti et al. [3], who identified PC and PE as altered serum metabolites in adults with the obesity–insulin resistance–T2DM phenotype. However, it remains unclear when these alterations occur (disease onset, progression, or throughout the course of metabolic deterioration).
In recent years, the common fruit fly (Drosophila melanogaster) has emerged as a valuable model system to study metabolism, insulin signaling, and DM. Its short life cycle and approximately sixty-day lifespan provide advantages over other animal models, especially in the context of longitudinal studies. The basic metabolic functions of fruit flies are evolutionarily conserved, including the maintenance of circulating sugar levels and storing excess energy in the form of lipids and glycogen. Key organ systems regulating nutrient uptake, storage, and metabolism are conserved between D. melanogaster and humans [7,8].
Several studies have demonstrated that high-calorie diets, such as diets rich in fructose, sucrose, and fats, promote triglyceride accumulation (obesity), hyperglycemia, and insulin resistance [9,10,11,12]. D. melanogaster has been proposed as an in vivo model for studying the onset, development, and treatment of T2DM [13]. Here we conducted a longitudinal lipidomic analysis to characterize metabolic alterations during the obesity–insulin resistance–T2DM transition using a high-sugar diet (HSD) D. melanogaster model.
2. Materials and Methods
2.1. Biological Material
The Oregon R (ORR) wild-type strain of D. melanogaster was used in all experiments and was originally obtained from John Carlson. Flies were maintained under standard conditions (25 °C, 50% relative humidity, and 12 h/12 h light/dark cycle) in 50 mL plastic tubes containing a standard diet medium.
2.2. Experimental Design
Newly hatched adult male flies (1-day old) were collected and maintained on a standard diet medium for three days. Then, flies were randomized into two groups, (i) standard diet (SD) and (ii) high-sugar diet (HSD), the latter designed to induce an obesity-related T2DM-like phenotype [14]. The SD consisted of 10% yeast, 9% fructose, 1.6% agar, 1.4% gelatin, and 1% propionic acid as a preservative. The HSD had a similar composition to the SD, except for an increased fructose content (30%). The nutritional composition of both diets was determined by AOAC methods and is shown in Table S1. The HSD showed significantly higher carbohydrate content and lower protein, ash, and moisture content compared to the SD.
Twenty flies were placed in each tube and transferred to fresh medium every six days. The experiment was conducted following the guidelines of the National Research Council’s Guide for the Care and Use of Laboratory Animals. The research protocol was approved by the Bioethics Committee of the Chemistry School of the Autonomous University of Querétaro (approval number: CBQ20/044, Querétaro, México).
2.3. Dietary Intake Estimation
To assess food intake, a modified CAFE (capillary feeder) assay was conducted as described by Diegelman et al. [15]. Briefly, male flies were housed in agarose-lined vials equipped with four micro-capillaries containing either SD or HSD liquid food. Intake was measured every 24 h and adjusted for evaporation using control vials without flies. We calculated cumulative consumption every 2-day period throughout the 14-day experiment.
2.4. Fly Body Weight Measurement
Fly body weight was recorded on days 0, 2, 4, 6, 8, 10, and 14. To accomplish this, five cold-anesthetized flies were transferred into a pre-weighed empty tube, weighed using an analytical balance, and the tube’s weight was subtracted. We calculated average body weight per group of five flies. Results are expressed in mg body weight per fly as the mean value of 10 biological replicates.
2.5. Fly Survival
Survival of flies fed SD and HSD was assessed at the end of the experiment. The number of dead flies per vial was counted, and the mean survival percentage was calculated for each group.
2.6. Total Triglyceride Measurement
Total triglycerides were measured in whole flies fed SD and HSD for 0, 2, 4, 6, 8, 10, 12, and 14 days. Five flies from each experimental group were anesthetized on ice, weighed, and homogenized in 100 μL of a phosphate-buffered saline solution containing 10.14 mM disodium hydrogen phosphate, 1.75 mM potassium dihydrogen phosphate, 137 mM NaCl, and 0.05% Tween 20. The homogenates were incubated at 70 °C for 5 min [16]. Then, 25 μL of the supernatant from each sample was mixed with 250 μL of triglyceride reagent (Triglycerides-LQ, Spinreact, Girona, Spain) in a microplate. Samples were incubated at 37 °C for 5 min, and absorbances were measured at 505 nm in a microplate spectrophotometer (Multiskan G0, Thermo Fisher Scientific, Waltham, MA, USA). Results are expressed as µg triglycerides per mg of body weight and represent the mean value of 10 biological replicates.
2.7. Hemolymph Combined Glucose + Trehalose Measurement
Total glucose and trehalose content was determined from the hemolymph of flies fed SD and HSD at 0, 2, 4, 6, 8, 10, 12, and 14 days. Forty-five flies from each experimental group were anesthetized on ice and carefully beheaded. The bodies were then collected in a 0.5 mL centrifuge tube with a perforated bottom, placed inside a 1.5 mL centrifuge tube. Bodies were centrifuged at 9000× g for 5 min at 4 °C to collect hemolymph in the outer tube. A 1 μL aliquot of hemolymph was collected and diluted with 99 μL of trehalase buffer containing 5 mM Tris, 137 mM sodium chloride, and 2.7 mM potassium chloride (pH 6.6). Samples were incubated at 70 °C for 5 min to inactivate endogenous trehalase. Then, an aliquot of 50 μL was mixed with 0.05 U of trehalase (T8778, Sigma-Aldrich, St. Louis, MO, USA) and incubated overnight at 37 °C. Trehalase-digested samples were then centrifuged at 9000× g for 5 min at 4 °C [16]. Finally, 30 μL of the supernatant was recovered and mixed with 250 μL of glucose reagent (Glucose-LQ, Spinreact, Sant Esteve de’n Bas, Girona, Spain) in a microplate, followed by incubation at 37 °C for 10 min. Absorbances were measured at 540 nm in a microplate spectrophotometer (Multiskan G0, Thermo Fisher Scientific, Waltham, MA, USA). Results are expressed as mg of trehalose + glucose per dL of hemolymph and represent the mean value of 5 biological replicates.
2.8. Ilp2 Relative Expression Assessment
The relative expression of Drosophila insulin-like peptide 2 (Ilp2) was measured in head samples obtained from flies fed SD and HSD at 0, 2, 4, 6, 8, 10, 12, and 14 days. Twenty flies from each experimental group were anesthetized on ice and beheaded for head collection. Total RNA was extracted with the Trizol reagent (Invitrogen, Waltham, MA, USA) following the manufacturer’s instructions. RNA integrity was assessed through 1% agarose gel electrophoresis, while RNA purity was evaluated by measuring the 260/280 and 260/230 ratios. RNA concentration was determined at 260 nm using a nanodrop spectrophotometer (Multiskan G0, Thermo Fisher Scientific). cDNA synthesis was carried out using 2 μg of RNA, 0.5 μL of oligodT primer (2 μg/mL), 1.25 μL of dNTP mix (10 mM Promega Co., Madison, WI, USA), 0.7 μL of RNAse inhibitor (Promega Co.), 1 μL of M-MLV RT (Promega Co.), and 5 μL M-MLV 5x reagent buffer (Promega Co.), in a final reaction volume of 25 μL. The reaction was incubated at 37 °C for 60 min in a thermal cycler (C100, Bio-Rad Laboratories, Hercules, CA, USA). Real-time PCR (RT-PCR) was carried out using 5 μL of cDNA, 2 μL of each primer at 10 mM, and 15 μL of the PCR mix (Roche, Baden-Württemberg, Germany). Samples were pre-incubated at 95 °C for 10 min, then amplified with 45 cycles of 95 °C for 10 s (denaturation), 54 °C for 10 s (primer alignment), and 72 °C for 10 s (elongation). Melting curves were obtained under the following conditions, 95 °C for 10 s, 65 °C for 10 s, and 97 °C for 10 s, using a RT-PCR system (LightCycler96, Roche, Basel, Switzerland). Negative controls were included to assess potential contamination. The following primers were used for the amplification of Ilp2: Fw, GTATGGTGTGCGAGGAGTAT and Rv, TGAGTACACCCCCAAGATAG; Act5C (reference gene): Fw, CACACCAAATCTTACAAAATGTGTGA and Rv, AATCCGGCCTTGCACATG. Ilp2 mRNA relative expression was normalized against Act5c using the 2-ΔΔCt method [17].
2.9. Feature-Based Targeted Lipidomic Analysis
Lipidomic analysis was carried out with whole flies fed SD and HSD at 0, 2, 4, 6, 8, 10, 12, and 14 days. Ten flies from each experimental group were anesthetized on ice and ground with liquid nitrogen. Then, polar metabolites were extracted with 1.6 mL of cold methanol/water (80:20 v/v) solution in an ultrasonic bath at 40 kHz for 10 min at room temperature (08895-75, Antylia Scientific, Vernon Hills, IL, USA). Samples were centrifuged at 5000× g for 15 min at 25 °C. The supernatants were recovered and concentrated under vacuum at 35 °C for 24 h (SpeedVac Savant, Thermo Scientific). Dried samples were resuspended in 200 μL of methanol and centrifuged at 5000× g for 10 min at 25 °C. Finally, supernatants were passed through PVDF syringe filters (0.45 μm, 13 mm) and collected into amber chromatographic vials with glass inserts. Samples were stored at −20 °C until further analysis.
Samples (1 μL) were injected with the full-loop mode into a BEH C18 Acquity column (2.1 mm × 100 mm, 1.7 μm) installed in an Ultra Performance Liquid Chromatograph (UPLC) coupled to a Quadrupole Time-of-Flight Mass Spectrometer (QTOF MSE) with an atmospheric pressure electrospray ionization source (Vion, Waters Co., Milford, MA, USA). Quality control (QC) samples were prepared by pooling an identical volume (1 μL) of each sample, whereas blank samples were prepared using the extraction solvents (methanol/water 80:20 v/v).
Three consecutive blank injections of the mobile phase were performed prior to the analytical sequence for system conditioning. QC and solvent blank samples were injected every 10 biological samples to ensure the stability of the analytical conditions. All samples were maintained in the autosampler at 4 °C, whereas the column was maintained at 35 °C throughout the analysis.
The mobile phase consisted of (A) water/formic acid (99:1 v/v) and (B) acetonitrile/formic acid (99:1 (v/v) at a flow rate of 0.4 mL min−1, under gradient conditions as reported by Álvarez-Rodríguez et al. [18]. The ionization was carried out in negative mode (ESI-) at 2 kV and 120 °C. MS spectra were collected at a mass range of 100–1500 Da, using a low collision energy of 5 eV and high collision energy of 15–45 eV in centroid mode with a duty cycle time of 0.2 s. Leucine-enkephalin (50 pg mL−1) was infused at a flow rate of 10 μL min−1 every 5 min for lock-mass correction.
Data were acquired in a non-targeted full-scan mode and subsequently processed using UNIFI software version 1.7 (Waters Co.) through a feature-based targeted extraction approach, with selected molecular and fragment ions with a mass error tolerance of <5 ppm. In addition, fragment ion patterns were matched against databases (Metlin, Lipidmaps, HMDB). Table S2 shows the identification parameters of all the identified compounds in whole male D. melanogaster, whereas Figure S2 shows the high and low collision energy MS spectra of the discriminant metabolites identified in this study. Data were normalized using total ion abundance to reduce systematic bias within the experiment. Then, data were square root-transformed and Pareto-scaled. Lipids were labeled following the nomenclature used by the LipidMaps database, without specifying the position of the carbon–carbon double bonds or the fatty acid chain attachment to the glycerol molecule, as these characteristics could not be distinguished in the fragmentation pattern.
2.10. Statistical Analysis
All data are reported as mean values ± standard deviation (s.d.). Outlier (>1.5 IQR) and extreme outlier (>3.0 IQR) values were identified using box-and-whisker plots. Only extreme values were excluded from the analysis to avoid distortion of central tendency measurements, which did not alter the significance or trend of the observed effects. The Kolmogorov–Smirnov test was applied to assess normality, and Levene’s test was used to evaluate variance homogeneity. Then, data were subjected to Dunnett’s test (parametric variables) or Wilcoxon signed-rank test (non-parametric variables). p < 0.05 differences were considered significant. All statistical analyses were performed using JMP software version 15.1 (JMP Statistical Discovery LLC, Greensboro, NC, USA). Principal Component Analysis (PCA) and Partial Least Square-Discriminant Analysis (PLS-DA) plots, as well as hierarchical clustering heatmaps (Euclidean distance), were constructed with lipidomic data at each sampling time (2, 4, 6, 8, 10, 12, and 14 days) and Variable Importance in the Projection (VIP) plots were obtained using the Metaboanalyst 5.0 online platform.
3. Results
3.1. High-Sugar Diet Induces Metabolic Alterations Associated with Obesity and T2DM-like Progression
As is consistent with previous reports [19,20], HSD-fed adult male flies exhibited increased total triglyceride levels (Figure 1A). Specifically, total triglycerides were significantly (p < 0.05) higher two days after the initiation of HSD feeding compared to SD-fed flies (1.51-fold), a change maintained throughout the 14-day experimental period (ranging from 1.25- to 1.69-fold; Figure 1A). Notably, this elevation occurred despite the absence of significant differences in body weight between SD- and HSD-fed flies (Figure S1A). Interestingly, SD-fed flies consistently exhibited higher food intake compared to HSD-fed flies, likely due to the lower caloric density of the SD (Figure S1B). At the end of the experiment (day 14), the survival rate of HSD-fed flies was 65%, markedly lower than the 82% observed in SD-fed flies.
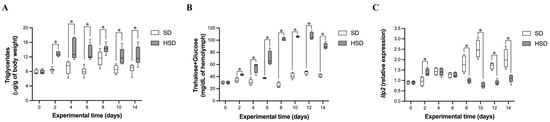
Figure 1.
Effect of high-sugar diet (HSD) on total triglyceride concentration (A), hemolymph trehalose + glucose concentration (B), and head Ilp2 relative expression (C) in male Drosophila melanogaster over 14 days. Data are shown as medians with interquartile ranges. * indicates statistically significant differences (p < 0.05) between groups (SD vs. HSD), as assessed by Dunnett’s or Wilcoxon signed-rank test. SD: standard diet; HSD: high-sugar diet.
Hemolymph combined trehalose and glucose concentrations were significantly (p < 0.05) elevated in HSD-fed flies from day 2 onwards, reaching 1.26- to 2.5-fold increases compared to SD-fed flies (Figure 1B). These levels plateaued between days 8 and 12, followed by a slight decrease on day 14. HSD-fed flies exhibited significantly (p < 0.05) higher Ilp2 expression levels compared to SD-fed flies at day 2 (1.4-fold; Figure 1C). However, no significant differences were observed between the experimental groups at days 4 and 6. From day 8 onward, Ilp2 expression was markedly lower in HSD-fed flies compared to SD-fed flies (1.82- to 3.25-fold, p < 0.05), which coincided with elevated hemolymph trehalose + glucose concentrations (Figure 1B), suggesting impaired carbohydrate clearance.
3.2. Targeted Lipidomic Profiling Identifies Early Metabolic Alterations During Obesity-Related Insulin Resistance and T2DM-like Progression
A targeted longitudinal lipidomic analysis was performed in whole male flies fed either SD or HSD over 14 days, focusing on fatty acids and phospholipids with carbon chain lengths of 16–24 carbons. Fold-changes (FCs) for all putatively identified metabolites are shown in Table 1. Asterisks (*) denote statistically significant differences (p < 0.05) between HSD- and SD-fed groups, regardless of FC magnitude. For biological interpretation, only lipid alterations that met both criteria, a FC > 1.3 or FC < −1.3 and p < 0.05, were considered relevant. As early as day 2 of HSD feeding, 11 metabolites were up-regulated and 11 down-regulated. These early lipidomic changes coincided with elevated total triglyceride levels, hemolymph trehalose+glucose concentration, and Ilp2 overexpression (Figure 1A, Figure 1B, and Figure 1C, respectively). To facilitate the identification of global patterns in lipid regulation, a heatmap of all detected lipid species was generated (Figure S3), in which up-regulated metabolites are shown in red and down-regulated metabolites are shown in blue.

Table 1.
Feature-based targeted lipidomic profile of male Drosophila melanogaster fed HSD compared to SD over 14 days.
To explore global lipidomic patterns, multivariate analyses were performed. The PCA and PLS-DA models based on the longitudinal targeted lipidome profile are shown in Figure 2A and Figure 2B, respectively. The unsupervised PCA model was used to assess the overall variance and natural clustering within the lipidomic dataset. The model explained 83.2% of total variance with the first two components, with PC1 accounting for 75.8%. The PCA revealed a clear separation between SD- and HSD-fed flies, particularly along PC1, suggesting that dietary intervention is a dominant factor driving lipidomic variation over time.
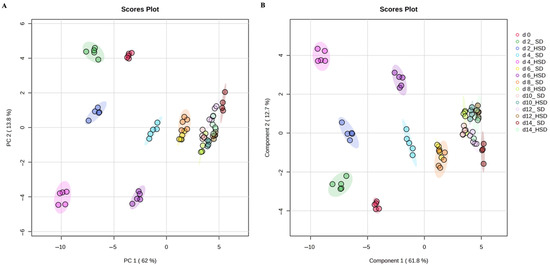
Figure 2.
Multivariate analyses of targeted longitudinal lipidome profile of SD- and HSD-fed male Drosophila melanogaster over 14 days. Principal Component Analysis (A) and Partial Least Squares-Discriminant Analysis (B) models of whole targeted lipidome profile of standard diet and high-sugar diet-fed male Drosophila melanogaster at 0, 2, 4, 6, 8, 10, 12 and 14 days. SD: standard diet; HSD: high-sugar diet; PC: principal component.
Then, a supervised PLS-DA model was obtained to enhance group discrimination by incorporating class labels in the analysis, which explained 81.4% of total variance, with PC1 contributing 74.5%. To ensure model robustness and avoid overfitting, the PLS-DA model was validated using 5-fold cross-validation to assess the classification performance (accuracy = 0.98, R2 = 0.96, and Q2 = 0.94 for two components) and permutation testing (n = 100) using separation distance to confirm model validity (p-Value < 0.01). This analysis confirmed the separation observed in the PCA and allowed for the identification of distinct lipidomic shifts associated with diet and disease progression. Interestingly, both models consistently showed that HSD-fed flies differentiated from SD-fed flies as early as day 2.
To further characterize these patterns, an unsupervised k-means clustering analysis was performed (Figure 3A). This analysis identified four distinct clusters based on lipidomic similarity, reflecting time-dependent changes in metabolic state. Figure 3B displays the relative levels of lipid species and associated metabolic parameters (body weight, triglyceride levels, trehalose + glucose concentration, and Ilp2 expression) across the four clusters identified by k-means analysis.
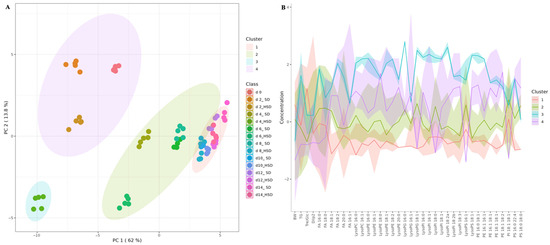
Figure 3.
K-means clustering model (A) and feature overview plot (B) based on targeted lipidome profile of SD- and HSD-fed male Drosophila melanogaster over 14 days. SD: standard diet; HSD: high-sugar diet; PC: principal component; BW: body weight; TG: triglycerides; Tre/Glc: trehalose + glucose; FA: fatty acid; PC: phosphatidylcholine; PE: phosphatidylethanolamine; PI: phosphatidylinositol; PG: phosphatidylglycerol; PS: phosphatidylserine.
Cluster 1 comprised flies at day 0 and flies fed either SD or HSD for 2 days, which exhibited intermediate levels of most lipid species, accompanied by the lowest body weight, triglyceride concentration, hemolymph trehalose + glucose content, and Ilp2 expression. Cluster 2 included exclusively flies fed HSD for 4 days, which showed the highest levels of triglycerides and of most individual lipid species. Cluster 3 grouped flies fed SD for 4, 6, and 8 days, together with flies fed HSD for 6 days, which exhibited moderate levels of all lipid species and metabolic parameters. Finally, Cluster 4 consisted of flies fed HSD for 8 days and flies fed either SD or HSD for 8, 10, 12, and 14 days, which showed overall low levels of individual lipid species and metabolic parameters. Together, these patterns illustrate a temporal and phenotypic progression that links lipidomic remodeling with metabolic dysfunction associated with HSD feeding.
Notably, HSD feeding for 4 days resulted in a lipidomic profile clearly distinguishable from that of SD-fed flies. Although the targeted lipidomic analysis did not fully discriminate among the obesity-associated insulin resistance, early T2DM-like, and late T2DM-like phenotypes in HSD-fed flies, major lipidomic alterations were still evident. Specifically, significant (p < 0.05) changes were detected at days 2 (11 up-regulated and 11 down-regulated), 4 (33 up-regulated and 2 down-regulated), and 6 (27 up-regulated and 1 down-regulated) of HSD feeding (Table 1), preceding the phenotypic development of obesity-related T2DM at day 8 (Figure 1C).
Given the extent of these early alterations, PLS-DA analyses were conducted at each experimental time point (Figure 4). Clear discrimination was observed between SD- and HSD-fed flies at every assessed time (days 2, 4, 6, 8, 10, 12, and 14). To identify the lipid species contributing most to this discrimination, VIP scores were calculated (Figure 4). These scores quantify the contribution of each metabolite to group separation within the PLS-DA model. Therefore, lipid species with high VIP scores represent key discriminants in the metabolic response due to HSD feeding. Notably, several lysophospholipids and monounsaturated fatty acids exhibited consistently high VIP scores across multiple time points, indicating their potential role as biomarkers of metabolic alterations.
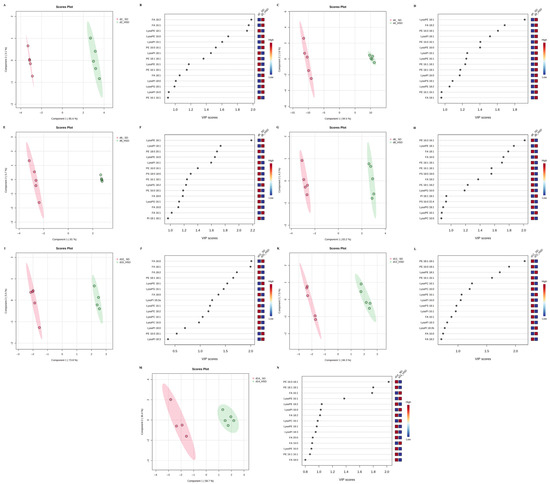
Figure 4.
Partial Least Square-Discriminant Analysis models and Variable Importance in the Projection plots of the targeted lipidome profile of SD- and HSD-fed male Drosophila melanogaster at 2 (A,B), 4 (C,D), 6 (E,F), 8 (G,H), 10 (I,J), 12 (K,L), and 14 (M,N) days. SD: standard diet; HSD: high-sugar diet; FA: fatty acid; PC: phosphatidylcholine; PE: phosphatidylethanolamine; PI: phosphatidylinositol; PG: phosphatidylglycerol; PS: phosphatidylserine.
LysoPE 18:1 was identified as one of the main discriminant metabolites between SD- and HSD-fed flies across days 2, 4, 6, 8, 10, and 12. At day 2, LysoPE 18:1 was significantly decreased in HSD-fed flies (−4.34-fold, p < 0.0001; Table 1; Figure 5E), followed by a significant increase from days 4 to 12 (1.59- to 8.42-fold, p < 0.0001). LysoPE 16:1 exhibited a similar dynamic pattern, showing a significant reduction at day 2 (−2.32-fold, p < 0.05; Table 1; Figure 5D) followed by increases at days 4 and 6 (2.34- and 2.55-fold, respectively, p < 0.05). PE 16:0 and 16:1 levels were significantly elevated (p < 0.0001) between days 2 and 6 in HSD-fed flies, with a notable decrease thereafter (Table 1; Figure 5F).
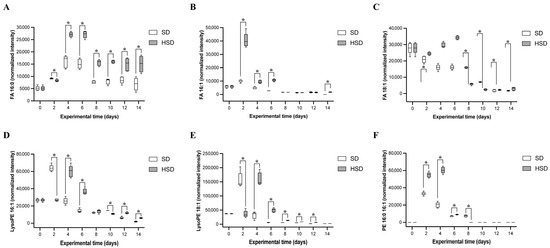
Figure 5.
Changes in discriminant lipid species in male Drosophila melanogaster fed SD or HSD over 14 days. FA 16:0 (A), FA 16:1 (B), FA 18:1 (C), LysoPE 16:1 (D), LysoPE 18:1 (E), and PE 16:0 16:1 (F) levels were determined every 2 days during the 14 days of HSD or SD. Data are shown as medians with interquartile ranges. * indicate statistically significant differences (p < 0.05) between experimental groups (SD vs. HSD), as assessed by Dunnett’s or Wilcoxon signed-rank test. SD: standard diet; HSD: high-sugar diet; FA: fatty acid; PE: phosphatidylethanolamine.
Palmitic acid (FA 16:0) increased significantly from day 4 to day 14 (1.68- to 2.17-fold, p < 0.01; Table 1; Figure 5A), peaking between days 4 and 6, following a similar trend to SD-fed flies but at higher levels. Palmitoleic acid (FA 16:1) was significantly elevated exclusively during the early phase of HSD induction (days 2–6), with the highest levels at day 2 (2.02- to 3.09-fold, p < 0.001; Table 1; Figure 5B). Oleic acid (FA 18:1) was markedly increased from day 2 to day 6 (3.54- to 14.07-fold, p < 0.01; Table 1; Figure 5C), but levels declined drastically afterward, becoming undetectable in both experimental groups by days 10–14.
4. Discussion
We hypothesized that a longitudinal targeted lipidomic analysis would enable the identification of distinctive metabolic features associated with obesity and T2DM-like phenotypes. Given that the transition from obesity to insulin resistance and T2DM in humans may span decades, we employed D. melanogaster as an in vivo model due to its low cost, short life cycle, and suitability for longitudinal metabolic studies [21].
In this study, HSD (30% fructose) administration induced an obesity-like phenotype in male flies, evidenced by increased total triglyceride levels from day 2, despite the absence of body weight changes. These findings are consistent with previous reports showing that high-fat (15%), -sucrose (30%), or -fructose (30%) diets induce hypercaloric diet-associated obesity in fruit flies [9,12]. Notably, the lack of weight gain and reduced survival observed in HSD-fed flies has been attributed previously to purine metabolism-induced dehydration, which shortens lifespan without affecting obesity, hyperglycemia, or insulin resistance [9,22].
Our results show that triglyceride accumulation is accompanied by hyperglycemia, as evidenced by elevated hemolymph trehalose + glucose levels from day 2. The Drosophila genome contains seven genes that encode insulin-like peptides (Ilps). Among them, Ilp2, Ilp3, and Ilp5 are synthesized and secreted by insulin producing cells (IPCs; median neurosecretory cells located in the dorsal brain) in a nutrition-dependent manner and are involved in glucose homeostasis [23]. Although Ilp2, Ilp3, and Ilp5 possess their own cis-regulatory elements, the expression of the three genes increases in a similar fashion in flies fed HSD and show comparable expression changes using foods with differing amounts of sugar [11,19,24]. To evaluate insulin production dynamics, we measured the expression of Ilp2 as a marker for IPC ILP production. Ilp2 is the gene most closely related to mature human insulin and is highly expressed in IPCs [25]. We found increased Ilp2 expression on day 2 in HSD-fed flies which, concurrent with hyperglycemia and elevated triglycerides, suggests early development of insulin resistance.
Between days 4 and 6, trehalose + glucose levels remained elevated in HSD-fed flies while Ilp2 expression remained unchanged, and from day 8 onward, Ilp2 expression was markedly reduced despite sustained hyperglycemia. This progression supports the interpretation of a temporal transition: an obesity-associated insulin resistance phenotype beginning at day 2 (hypertriglyceridemia, hyperglycemia, and Ilp2 over-regulation), followed by an early T2DM-like state at days 4–6 (persistent metabolic alterations with normal Ilp2 expression), and a late T2DM-like phenotype from days 8–14 (hypertriglyceridemia, hyperglycemia, and Ilp2 down-regulation). Other longitudinal studies modeling diet-induced T2DM in D. melanogaster remain scarce. Hong et al. [10] reported that high-fat diet feeding induced early hypertriglyceridemia and hyperinsulinemia by day 2, but hyperglycemia only emerged by day 6. These discrepancies underscore the diet-dependent nature of metabolic dysfunction [9].
To identify metabolic fingerprints associated with these phenotypes, we conducted a targeted lipidomic analysis focusing on 16–24 carbon long-chain fatty acids and phospholipids. To our knowledge, this is the first longitudinal lipidomic profiling of a hypercaloric diet-induced T2DM-like phenotype in D. melanogaster. The combination of targeted lipidomics and chemometric analyses enabled the stratification of experimental groups into distinct metabolic clusters. Although clustering was influenced by age, clear lipidomic distinctions between SD- and HSD-fed flies were observed at days 4 and 6, indicating critical windows for metabolic reprogramming. Discriminant analysis further identified three fatty acids (16:0, 16:1, and 18:1) and three PEs (16:1, 18:1, and 16:0 16:1) as key metabolites differentiating dietary groups.
Most alterations in these lipid species occurred between days 2 and 6, suggesting a metabolic shift preceding T2DM-like dysfunction. Only palmitic acid and oleic acid-containing LysoPE remained elevated through day 14, suggesting a role in the transition from insulin resistance to a T2DM-like state. Previous studies have demonstrated that palmitic acid (FA 16:0) is involved in metabolic dysfunction through mechanisms involving oxidative stress, pro-inflammatory cytokine production, and lipotoxicity-induced β-cell impairment [26]. Additionally, C16-derived phospholipids have been linked to endoplasmic reticulum stress, inflammation, and apoptosis [27].
Palmitoleic acid (FA 16:1) has been proposed as a beneficial lipokine associated with improved insulin sensitivity and β-cell function [28]. However, epidemiological studies have reported a positive association between circulating levels of palmitoleic acid, along with palmitic and oleic acids and increased T2DM risk, independent of obesity and other confounding variables [29], highlighting the complexity of interpreting their roles across physiological contexts.
The role of LysoPEs in metabolic diseases is an emerging area of interest. Kim et al. [30] reported elevated LysoPE species (16:0, 18:1, and 18:2) in metabolic unhealthy adults with overweight and atherogenic dyslipidemia and increased phospholipase A2 activity, suggesting enhanced PE hydrolysis and pro-atherogenic remodeling. Furthermore, exogenous LysoPE 18:2 was shown to promote non-alcoholic hepatic steatosis and steatohepatitis, conditions closely linked to insulin resistance [31]. Notably, LysoPE 18:1 was identified in our study as a key discriminant metabolite, consistent with prior findings in obese, hyperlipidemic, and insulin-resistance human cohorts [32].
Our findings highlight the complex and contradictory roles of specific lipid species in metabolic regulation. For instance, while palmitoleic acid has been described as a beneficial lipokine, elevated circulating levels have also been associated with increased hepatic lipogenesis and cardiovascular risk in insulin-resistant individuals [33]. Likewise, oleic acid, often considered metabolically protective due to its ability to enhance insulin signaling and glucose uptake, has also been associated with increased endogenous synthesis via stearoyl-CoA desaturase-1 (SCD1) in skeletal muscles, a condition that contributes to impaired fatty acid oxidation and lipid accumulation in individuals with obesity [34].
Notably, although triglycerides are widely recognized as key indicators of metabolic dysfunction, their persistent elevation may reflect a downstream consequence of metabolic overload. In contrast, the dynamic changes observed in LysoPE species support the hypothesis that phospholipid remodeling may act as an early mediator of insulin resistance [35]. However, the precise functional roles of these lipids in insulin signaling and glucose metabolism remain unclear.
While our study provides novel insight into temporal dynamics of lipidomic alterations in diet-induced T2DM-like pathology, limitations remain regarding the interpretation of mechanistic causality. Given the relatively recent application of lipidomics in metabolic research [36], further studies are needed to elucidate the functional implications of saturated and monounsaturated C16 and C18 lipids, particularly those incorporated into phospholipids, during the onset and progression of obesity, insulin resistance, and T2DM. Although D. melanogaster offers a genetically and metabolically conserved model, its anatomical differences with mammals limit the extrapolation of mechanistic findings. Nevertheless, the reproducibility of diet-induced phenotypes and lipidomic shifts supports its utility for investigating early metabolic dysregulation under controlled experimental conditions. In future studies, functional validation of the most discriminant lipid biomarkers, such as LysoPE 18:1 and FA 16:0, should be assessed to determine their causal contribution to metabolic transitions, clarify their role in the development of insulin resistance and T2DM-like states, and ultimately support the translational relevance of these findings into human health.
5. Conclusions
This study demonstrates that combining D. melanogaster as an in vivo model with a longitudinal targeted lipidomic approach enables the identification of early lipidomic alterations associated with diet-induced metabolic dysfunction. Key lipid species were significantly altered during the initial stages of HSD-induced obesity and insulin resistance and early T2DM (days 2 to 6), prior to the progression to a late T2DM-like phenotype (days 8 to 14). Notably, palmitic acid and oleic acid-containing LysoPE were persistently elevated from early time points through the development of the T2DM-like state, suggesting their potential involvement in disease progression. Further mechanistic studies are necessary to elucidate the functional roles of these lipid species in the onset and transition of metabolic disorders.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/diabetology6090092/s1. Figure S1: Effect of high-sugar diet (HSD) in male Drosophila melanogaster on body weight (A) and diet intake (B) over 14 days. Figure S2: High resolution MSE spectra at high (superior) and low (inferior) collision energy of discriminant metabolites between male Drosophila melanogaster fed high-sugar diet or standard diet over 14 days: FA 16:0 (A), FA 16:1 (B), FA 18:1 (C), LysoPE 16:1 (D), LysoPE 18:1 (E), and PE 16:0 16:1 (F). MS: mass spectra; FA: fatty acid; PE: phosphatidylethanolamine. Figure S3: Heatmap of feature-based targeted lipidomic profile of male Drosophila melanogaster fed HSD compared to SD over 14 days. Table S1: Proximal composition of standard and high-sugar diets fed to Drosophila melanogaster. Table S2: Parameters of putatively identified compounds by UPLC-QTOF MSE feature-based oriented lipidomic analysis of male Drosophila melanogaster.
Author Contributions
Conceptualization, J.M.M.-M. and I.F.P.-R.; formal analysis, A.D.B.-J., J.M.M.-M. and I.F.P.-R.; investigation, S.E.-N.; resources, D.G.G.-G., R.R.-C., J.R.R.-E., J.M.M.-M. and I.F.P.-R.; writing—original draft preparation, J.M.M.-M. and I.F.P.-R.; writing—review and editing, S.E.-N., D.G.G.-G., A.D.B.-J., R.R.-C. and J.R.R.-E.; supervision, J.M.M.-M. and I.F.P.-R.; project administration, S.E.-N., J.M.M.-M. and I.F.P.-R.; funding acquisition, S.E.-N. and I.F.P.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by ‘Fondo Química Somos Todos 2020’ of the Chemistry School of the Universidad Autónoma de Querétaro and by the ‘Fondo para Proyectos Especiales de Rectoría (FOPER) 2020’ of the Universidad Autónoma de Querétaro (FOPER-2020-FQU01887).
Institutional Review Board Statement
The animal study protocol was approved by the Bioethics Committee of the School of Chemistry of the Universidad Autónoma de Querétaro (CBQ20/044 on 27 May 2020).
Informed Consent Statement
Not applicable.
Data Availability Statement
Dataset available on request from authors.
Acknowledgments
The authors are grateful to CONACYT for the scholarships granted to S.E.-N. We thank the Chemistry Faculty of the Universidad Autónoma de Querétaro for providing facilities for the UPLC-Q-ToF MS analysis (CONACyT project number: INFR-15-255182), as well as the Clinical Service Unit and the Molecular Diagnosis Unit of the Chemistry Faculty of the Universidad Autónoma de Querétaro for providing the facilities for the biochemistry and molecular biology analyses. We are grateful to Brenda Ugalde Villanueva for the technical assistance in the molecular biology analysis and Flor González Cataño for the technical assistance in the UPLC-Q-ToF MSE analysis.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| T2DM | Type 2 diabetes mellitus |
| HSD | High-sugar diet |
| SD | Standard diet |
| PE | Phosphatidylethanolamine |
| LysoPE | Lysophosphatidylethanolamine |
| PI | Phosphatidylinositol |
| LysoPI | Lysophosphatidylinositol |
| PC | Phosphatidylcholine |
| PS | Phosphatidylserine |
| PG | Phosphatidylglycerol |
| FA | Fatty acid |
| CAFE | Capillary feeder |
| Ilp2 | Insulin-like peptide 2 |
| RT-PCR | Real-time polymerase chain reaction |
| QC | Quality control |
| UPLC | Ultra performance liquid chromatography |
| QToF | Quadrupole Time-of-flight |
| MS | Mass spectrometer |
| ESI | Electrospray ionization |
| PCA | Principal component analysis |
| PLS-DA | Partial least square-discriminant analysis |
| VIP | Variable importance in the projection |
| IPC | Insulin producing cells |
References
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- Wali, J.A.; Solon-Biet, S.M.; Freire, T.; Brandon, A.E. Macronutrient determinants of obesity, insulin resistance and metabolic health. Biology 2021, 10, 336. [Google Scholar] [CrossRef]
- Al-Sulaiti, H.; Diboun, I.; Agha, M.V.; Mohamed, F.F.; Atkin, S.; Dömling, A.S.; Elrayess, M.A.; Mazloum, N.A. Metabolic signature of obesity-associated insulin resistance and type 2 diabetes. J. Transl. Med. 2019, 17, 348. [Google Scholar] [CrossRef]
- Wondmkun, Y.T. Obesity, insulin resistance, and type 2 diabetes: Associations and therapeutic implications. Diabetes Metab. Syndr. Obes. 2020, 13, 3611. [Google Scholar] [CrossRef]
- Vanamala, J.K.; Sivaramakrishnan, V.; Mummidi, S. Integrated multi-omic studies of metabolic syndrome, diabetes and insulin-related disorders: Mechanisms, biomarkers, and therapeutic targets. Front. Endocrinol. 2025, 15, 1537554. [Google Scholar] [CrossRef]
- Palau-Rodriguez, M.; Marco-Ramell, A.; Casas-Agustench, P.; Tulipani, S.; Minñarro, A.; Sanchez-Pla, A.; Murri, M.; Tinahones, F.J.; Andres-Lacueva, C. Visceral adipose tissue phospholipid signature of insulin sensitivity and obesity. J. Proteome Res. 2021, 20, 2410–2419. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Rendón, J.P.; Salceda, R.; Riesgo-Escovar, J.R. Drosophila melanogaster as a model for diabetes type 2 progression. Bimed Res. Int. 2018, 2018, 1417528. [Google Scholar]
- Murillo-Maldonado, J.M.; Riesgo-Escovar, J.R. Development and diabetes on the fly. Mech. Dev. 2017, 144, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Baenas, N.; Wagner, A.E. Drosophila melanogaster as a model organism for obesity and type-2 diabetes mellitus by applying high-sugar and high-fat diets. Biomolecules 2022, 12, 307. [Google Scholar] [CrossRef]
- Hong, S.H.; Kang, M.; Lee, K.S.; Yu, K. High fat diet-induced TGF-β/Gbb signaling provokes insulin resistance through the tribbles expression. Sci. Rep. 2016, 6, 30265. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.N.S.; Coogan, C.; Chamseddin, K.; Fernandez-Kim, S.O.; Kolli, S.; Keller, J.N.; Bauer, J.H. Development of diet-induced insulin resistance in adult Drosophila melanogaster. Biochim. Biophys. Acta Mol. Basis Dis. 2012, 1822, 1230–1237. [Google Scholar] [CrossRef]
- Musselman, L.P.; Fink, J.L.; Baranski, T.J. Similar effects of high-fructose and high-glucose feeding in a Drosophila model of obesity and diabetes. PLoS ONE 2019, 14, e0217096. [Google Scholar] [CrossRef]
- Musselman, L.P.; Kühnlein, R.P. Drosophila as a model to study obesity and metabolic disease. J. Exp. Biol. 2018, 221, jeb163881. [Google Scholar] [CrossRef]
- Rovenko, B.M.; Perkhulyn, N.V.; Gospodaryov, D.V.; Sanz, A.; Lushchak, V.; Lushchak, V.I. High consumption of fructose rather than glucose promotes a diet-induced obese phenotype in Drosophila melanogaster. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2015, 180, 75–85. [Google Scholar] [CrossRef]
- Diegelmann, S.; Jansen, A.; Jois, S.; Kastenholz, K.; Velo Escarcena, L.; Strudthoff, N.; Scholz, H. The CApillary FEeder assay measures food intake in Drosophila melanogaster. J. Vis. Exp. 2017, 17, 55024. [Google Scholar]
- Tennessen, J.M.; Barry, W.E.; Cox, J.; Thummel, C.S. Methods for studying metabolism in Drosophila. Methods 2014, 68, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2011, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Rodríguez, I.I.; Castaño-Tostado, E.; García-Gutiérrez, D.G.; Reynoso-Camacho, R.; Elton-Puente, J.E.; Barajas-Pozos, A.; Pérez-Ramírez, I.F. Non-targeted metabolomic analysis reveals serum phospholipid alterations in patients with early stages of diabetic foot ulcer. Biomark. Insights 2020, 15, 1177271920954828. [Google Scholar] [CrossRef] [PubMed]
- Musselman, L.P.; Fink, J.L.; Narzinski, K.; Ramachandran, P.V.; Sukumar Hathiramani, S.; Cagan, R.L.; Baranski, T.J. A high-sugar diet produces obesity and insulin resistance in wild-type Drosophila. Dis. Model. Mech. 2011, 4, 842–849. [Google Scholar] [CrossRef]
- Skorupa, D.A.; Dervisefendic, A.; Zwiener, J.; Pletcher, S.D. Dietary composition specifies consumption, obesity, and lifespan in Drosophila melanogaster. Aging Cell 2008, 7, 478–490. [Google Scholar] [CrossRef]
- Meshrif, W.S.; El Husseiny, I.M.; Elbrense, H. Drosophila melanogaster as a low-cost and valuable model for studying type 2 diabetes. J. Exp. Zool. A Ecol. Integr. Physiol. 2022, 337, 457–466. [Google Scholar] [CrossRef]
- van Dam, E.; van Leeuwen, L.A.; Dos Santos, E.; James, J.; Best, L.; Lennicke, C.; Vincent, A.J.; Marinos, G.; Foley, A.; Buricova, M.; et al. Sugar-induced obesity and insulin resistance are uncoupled from shortened survival in Drosophila. Cell Met. 2020, 31, 710–725. [Google Scholar] [CrossRef]
- Inoue, Y.H.; Katsube, H.; Hinami, Y. Drosophila models to investigate insulin action and mechanisms underlying human diabetes mellitus. In Drosophila Models for Human Diseases; Yamaguchi, M., Ed.; Springer: Singapore, 2018; Volume 1075, pp. 235–256. [Google Scholar]
- Ikeya, T.; Galic, M.; Belawat, P.; Nairz, K.; Hafen, E. Nutrient-dependent expression of insulin-like peptides from neuroendocrine cells in the CNS contributes to growth regulation in Drosophila. Curr. Biol. 2002, 12, 1293–1300. [Google Scholar] [CrossRef]
- Brogiolo, W.; Stocker, H.; Ikeya, T.; Rintelen, F.; Fernandez, R.; Hafen, E. An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Curr. Biol. 2001, 11, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mao, Y.S.; Chen, F.; Xia, D.X.; Zhao, T.Q. Palmitic acid up regulates Gal-3 and induces insulin resistance in macrophages by mediating the balance between KLF4 and NF-κB. Exp. Ther. Med. 2021, 22, 1028. [Google Scholar] [CrossRef] [PubMed]
- Korbecki, J.; Bajdak-Rusinek, K. The effect of palmitic acid on inflammatory response in macrophages: An overview of molecular mechanisms. Inflamm. Res. 2019, 68, 915–932. [Google Scholar] [CrossRef]
- Tricò, D.; Mengozzi, A.; Nesti, L.; Hatunic, M.; Gabriel Sanchez, R.; Konrad, T.; Lalić, K.; Lalić, N.M.; Mari, A.; Natali, A. Circulating palmitoleic acid is an independent determinant of insulin sensitivity, beta cell function and glucose tolerance in non-diabetic individuals: A longitudinal analysis. Diabetologia 2020, 63, 206–218. [Google Scholar] [CrossRef]
- Qureshi, W.; Santaren, I.D.; Hanley, A.J.; Watkins, S.M.; Lorenzo, C.; Wagenknecht, L.E. Risk of diabetes associated with fatty acids in the de novo lipogenesis pathway is independent of insulin sensitivity and response: The Insulin Resistance Atherosclerosis Study (IRAS). BMJ Open Diabetes Res. Care 2019, 7, e000691. [Google Scholar] [CrossRef]
- Kim, M.; Yoo, H.J.; Ko, J.; Lee, J.H. Metabolically unhealthy overweight individuals have high lysophosphatide levels, phospholipase activity, and oxidative stress. Clin. Nutr. 2020, 39, 1137–1145. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Sakurai, T.; Chen, Z.; Inoue, N.; Chiba, H.; Hui, S.P. Lysophosphatidylethanolamine affects lipid accumulation and metabolism in a human liver-derived cell line. Nutrients 2022, 14, 579. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, D.; Li, Y.; Guo, L.; Cui, Y.; Zhang, X.; Li, E. Metabolomic analysis of serum from obese adults with hyperlipemia by UHPLC-Q-TOF MS/MS. Biomed. Chromatogr. 2016, 30, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Frigolet, M.E.; Gutiérrez-Aguilar, R. The role of the novel lipokine palmitoleic acid in health and disease. Adv. Nutr. 2017, 8, 173S–181S. [Google Scholar] [CrossRef] [PubMed]
- Hulver, M.W.; Berggren, J.R.; Carper, M.J.; Miyazaki, M.; Ntambi, J.M.; Hoffman, E.P.; Thyfault, J.P.; Stevens, R.; Dohm, G.L.; Houmard, J.A.; et al. Elevated stearoyl-CoA desaturase-1 expression in skeletal muscle contributes to abnormal fatty acid partitioning in obese humans. Cell Metab. 2005, 2, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.J.; Liu, T.T.; Pan, J.C.; Man, Q.Q.; Song, S.; Zhang, J. The Association between the plasma phospholipid profile and insulin resistance: A population-based cross-section study from the China Adult Chronic Disease and Nutrition Surveillance. Nutrients 2024, 16, 1205. [Google Scholar] [CrossRef] [PubMed]
- Hosseinkhani, S.; Aazami, H.; Hashemi, E.; Dehghanbanadaki, H.; Adibi-Motlagh, B.; Razi, F. The trend in application of omics in type 2 diabetes researches; A bibliometric study. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 102250. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).