Abstract
Youth-onset type 2 diabetes (T2D) is a growing public health challenge. This narrative review aims to provide a comprehensive overview of epidemiology, pathophysiology, diagnosis, complications, and therapeutic strategies in children and adolescents with T2D, highlighting the most recent evidence and the distinctive features that differentiate youth-onset from adult-onset disease. Over recent decades, its incidence has increased worldwide, closely linked to rising rates of childhood obesity, sedentary behavior, and socioeconomic disparities. The disease typically emerges around puberty, a period marked by physiological insulin resistance, and is influenced by a complex interplay of genetic, environmental, and developmental factors. Diagnosis can be delayed or missed due to overlapping features with type 1 diabetes and limitations in current screening tools. The clinical course is often aggressive, with early onset of microvascular and macrovascular complications. Management is particularly challenging due to the limited number of pharmacologic agents approved for pediatric use and the psychological and behavioral complexities of adolescence. While metformin remains the first-line treatment, newer therapies such as GLP-1 receptor agonists and SGLT2 inhibitors show promise in improving metabolic outcomes. In conclusion, early diagnosis, multidisciplinary management, and equitable access to effective therapies are essential to improve long-term outcomes in this vulnerable population.
1. Introduction
Type 2 diabetes (T2D) is a complex metabolic disorder characterized by the progressive decline in pancreatic β-cell function in the context of peripheral insulin resistance [1]. Historically considered as an adult-onset disease, the incidence of T2D among pediatric populations has risen at an alarming rate, paralleling the global increase in childhood obesity and sedentary lifestyles [2,3,4,5].
Youth-onset T2D exhibits distinct pathophysiological mechanisms compared to its adult counterpart, including a heightened degree of insulin resistance, accelerated β-cell dysfunction, and an increased susceptibility to early-onset microvascular and macrovascular complications [6,7,8,9].
The pathogenesis of pediatric T2D is driven by a complex interplay of genetic susceptibility, ethnic predisposition, and environmental factors, such as excessive caloric intake and insufficient physical activity [10]. Furthermore, metabolic dysregulation—characterized by ectopic lipid accumulation, chronic low-grade inflammation, and adipose tissue dysfunction—exacerbates insulin resistance and further increases functional demand on β-cells, accelerating disease progression [11].
Puberty represents a critical window for the manifestation of T2D due to transient physiological insulin resistance, which is typically counterbalanced by adaptive increases in basal and glucose-stimulated insulin secretion [12]. While insulin sensitivity is generally restored post-puberty [13], obese adolescents often exhibit persistent hyperinsulinemia and dysglycemia. Chronic hyperglycemia contributes to β-cell dysfunction through glucotoxicity and lipotoxicity, promoting oxidative stress, endoplasmic reticulum dysfunction, and ultimately β-cell apoptosis, thereby further compromising insulin homeostasis [14].
In recent years, youth-onset T2D has emerged as a major public health concern due to its rising prevalence and aggressive clinical course. Management is further complicated by the limited availability of approved therapies for pediatric use and the challenges of treatment adherence during adolescence, leading to suboptimal outcomes.
This narrative review aims to provide an in-depth analysis of the epidemiology, risk factors, clinical presentation, and management strategies in children and adolescents with T2D, highlighting the most recent evidence and the distinctive features that differentiate youth-onset from adult-onset disease.
2. Epidemiology
Pediatric diabetes is an escalating global public health concern. Over the past two decades, the incidence of T2D during childhood and adolescence have risen significantly, paralleling the global surge in obesity rates [15]. Projections indicate that, if current trends persist, the prevalence of youth-onset T2D could quadruple by 2050 [16].
In 2021, approximately 41,600 new pediatric cases of T2D were diagnosed globally. As indicated by the International Diabetes Federation (IDF), the Western Pacific region accounted for 30% of these cases, while 40% were reported in upper-middle-income countries. China, India, and the United States reported the highest incidence rates [17]. Conversely, the lowest incidence rates were observed among non-Hispanic White youth in the United Kingdom, Germany, and the United States [18,19,20]. These differences likely reflect a complex interplay of genetic susceptibility, environmental and socioeconomic factors, as well as variability in screening and diagnostic practices across countries and ethnic groups, which may lead to underdiagnosis in certain settings.
A summary of key epidemiological studies reporting incidence and prevalence of youth-onset T2D across different regions is provided in Table 1.

Table 1.
Incidence and prevalence of youth-onset type 2 diabetes across different countries and populations.
The SEARCH for Diabetes in Youth Study, a population-based surveillance study monitoring physician-diagnosed diabetes across six U.S. states and selected American Indian reservations, reported that the incidence of T2D among individuals aged 10 to 19 years increased from 9 cases per 100,000 person-years in 2002–2003 to 12.5 cases per 100,000 person-years in 2011–2012. This trend was consistently observed across all demographic subgroups, including age, sex, race, and ethnicity. The estimated prevalence of youth-onset T2D among individuals aged 10–19 years currently stands at 0.67 per 1000 individuals, an increase from 0.34 per 1000 in 2001, representing a relative rise of 95.3% over 16 years [21]. African American (0.85 per 1000; 95% CI, 0.74–0.97) and Hispanic (0.57 per 1000; 95% CI, 0.51–0.64) youth exhibited the highest absolute increases during 2001–2017 [22].
A study from England analyzing data of the National Paediatric Diabetes Audit identified 122,780 individuals under 40 years with T2D, representing 4.6% of the total T2D population. Among these, 650 (0.5%) were under 16 years, 910 (0.7%) were aged 16–18 years, 8245 (6.7%) were aged 19–25 years, and 112,975 (92%) were aged 26–39 years. Compared to individuals over 40 years old, younger patients exhibited female predominance and a significantly higher prevalence of ethnic minority backgrounds, obesity, and socioeconomic disadvantage [23].
An Indian study analyzing data from the Comprehensive National Nutrition Survey documented a prediabetes/T2D prevalence of 12.3% among adolescent males and 8.4% among adolescent females. Body Mass Index (BMI) and subscapular skinfold thickness were identified as the most significant predictors of prediabetes and diabetes, while physical activity showed a protective effect. Adolescent females from socioeconomically disadvantaged backgrounds exhibited a significantly higher prevalence of T2D [20]. Similarly, the Treatment Options for Type 2 Diabetes in Adolescents & Youth (TODAY) study reported that approximately 40% of affected families had an annual household income of $25,000 or less, suggesting a strong link between socioeconomic status and clinical outcomes [24].
Recent regional studies further emphasize the growing burden of T2D, highlighting significant increases in incidence and prevalence across different populations. In North Rhine-Westphalia, Germany, incidence rates increased from 1.3 to 2.8 per 100,000 person-years between 2002 and 2022 [25]. In Mexico, the proportion of pediatric diabetes due to T2D increased from 20.2% to 33.0% over 5 years [26]. Saudi Arabia’s Pediatric and Youth Diabetes Registry, comprising 2344 individuals, reports that 6.4% has T2D [27].
A retrospective study at Barts Health NHS Trust in London documented a doubled incidence of T2D, from 2.6 cases per year (2008–2013) to 5.4 cases per year (2014–2018) [28]. In Israel, incidence increased from 0.63 per 100,000 in 2008 to 3.41 per 100,000 in 2019 [29]. In Manitoba, Canada, T2D incidence nearly doubled from 16.0 to 31.1 per 100,000 per year between 2009–2010 and 2017–2018, with the most pronounced rise among First Nations children [30].
The COVID-19 pandemic has further exacerbated the pediatric T2D crisis. A significant increase in the incidence of T2D during this period has been recorded. It remains uncertain whether this rise was attributable to SARS-CoV-2 itself or to pandemic-related behavioral changes [31]. A multicenter retrospective study conducted across 24 hospital-based centers in the US analyzed data from youth aged ≤21 years who were newly diagnosed with T2D between March 2018 and February 2021. The findings revealed a 77.2% increase in new T2D diagnoses during the first year of the pandemic compared to the average of the previous two years. Additionally, the likelihood of presenting with metabolic decompensation and severe diabetic ketoacidosis significantly increased during the pandemic [32].
The underlying causes of this surge are likely multifactorial. SARS-CoV-2 has been hypothesized to increase hyperglycemia risk by interacting with angiotensin-converting enzyme 2 (ACE2) receptors expressed in key metabolic organs and tissues, including pancreatic β-cells [31]. However, the rising incidence of T2D may also be attributed to pandemic-related behavioral changes, such as reduced physical activity and increased weight gain. Indeed, studies have reported that the rate of BMI increase in children nearly doubled during the pandemic compared to pre-pandemic levels [33].
Epidemiological evidence from multiple studies corroborates this trend. A study conducted at the Children’s Hospital of Chicago involving patients under 21 years of age reported that, prior to the COVID-19 pandemic, the mean annual incidence of newly diagnosed T2D cases was 54.2. During the pandemic period, this figure rose sharply to 159 cases per year, representing a 193% increase relative to the pre-pandemic baseline [34]. Similarly, in Germany, the incidence of T2D among individuals aged 6 to 18 years increased by 188% during the second year of the pandemic compared to preceding years [35].
In addition to the increase in new cases, the severity of clinical presentation worsened. Patients diagnosed with T2D during the pandemic exhibited higher initial glycated hemoglobin (HbA1c) levels and an increased prevalence of diabetic ketoacidosis at diagnosis. These trends may be attributed to delays in seeking medical care, and limited access to healthcare services [36].
3. Risk Factors
The development of T2D results from a complex interplay of genetic, epigenetic, and environmental factors. Although the etiopathogenesis of T2D in pediatric age remains partially unknown, several well-established risk factors have been identified.
Obesity is a fundamental modifiable risk factor for the onset of T2D, acting on the pathophysiology of insulin resistance and metabolic dysfunction [11]. A retrospective cohort study conducted on 369,362 participants aged 2 to 15 years revealed a fourfold increased risk of developing T2D among overweight or obese individuals compared to those with a normal BMI [37].
Adolescence is a crucial developmental phase marked by hormonal and psychological changes and at high incidence of obesity [12]. The considerable prevalence of overweight especially among adolescents is mainly related to pre-packaged foods consumption and high-calorie diets. During this developmental stage, dietary habits often deteriorate due to increased autonomy in food choices and greater exposure to ultra-processed foods.
In addition, poor activity lifestyle and sedentary behaviors amplify the risk of sustained weight gain and metabolic dysregulation among young males and females. A five-year longitudinal study involving 2516 adolescents highlights a gradual increase in leisure-time computer use, particularly among boys, from 11.4 to 15.2 h per week between early and mid-adolescence, and from 10.4 to 14.2 h per week between mid- and late adolescence [38]. Conversely, an observational study conducted on a cohort of 686 youth from Belgium, Greece, Hungary, the Netherlands, and Switzerland revealed that girls spent higher sedentary time (500 min/day) compared to boys (474 min/day) [39].
Even though BMI is traditionally considered as a marker of obesity, the distribution of adipose tissue is now acknowledged as a key indicator of metabolic risk. The excess of perivisceral fat depots predicts the development of cardiometabolic abnormalities, insulin-resistance, and T2D in children. A cohort study conducted on 520 young Japanese Americans without diabetes, identified body fat deposition as a significant predictor of eventual T2D onset [40].
Perihepatic and epicardial adipose tissue has also been associated with increased secretion of pro-inflammatory cytokines (TNF-α, IL-6) and reduced levels of insulin-sensitizing adipokines, such as adiponectin, resulting in impaired glucose intracellular uptake and increased susceptibility to T2D among obese adolescents [41].
Puberty is the physiological transition from childhood to adulthood, characterized by the maturation of the reproductive system. Females exhibit a higher susceptibility to T2D during puberty compared to males. This difference is probably associated with a combination of genetic, hormonal, and metabolic factors. Puberty usually occurs earlier in girls, and is associated with lower insulin sensitivity compared to boys at the same Tanner stage. However, following puberty, males face a greater risk of developing insulin resistance compared to females [42].
Prenatal conditions may also play a crucial role in shaping fetal metabolic programming and are significant contributors to the rising prevalence of youth-onset T2D. Maternal factors such as gestational diabetes and obesity can induce long-lasting alterations in the offspring’s metabolic pathways through epigenetic modifications. Understanding the impact of prenatal influences on metabolic health highlights the necessity for early preventive strategies to mitigate the intergenerational transmission of metabolic disorders [43].
A retrospective case–control study investigated the associations between prenatal, obstetric, and perinatal factors and the risk of developing youth-onset T2D, revealing that exposure to maternal pregestational diabetes increases the risk by nearly sixfold, while exposure to gestational diabetes is associated with a fourfold elevated risk. Additionally, the study identified breastfeeding as a protective factor against the development of T2D in youth [44].
Moreover, individuals with intrauterine growth restriction and those born small for gestational age may develop what is referred to as the “thrifty phenotype”. According to this hypothesis, intrauterine malnutrition serves as a trigger for fetal adaptive mechanisms that are essential for short-term survival. However, these adaptations may also lead to long-term metabolic consequences, including an increased risk of insulin resistance and the subsequent early development of T2D [45].
Insufficient sleep has been identified as a contributing risk factor to the development of unhealthy dietary patterns, insulin resistance, and impaired glucose tolerance, particularly among adolescents. Furthermore, chronic sleep deprivation may exacerbate the difficulties in maintaining optimal blood glucose levels in children and adolescents already diagnosed with diabetes. A study enrolling 551 individuals with T2D revealed lower sleep quality assessed through the Pittsburgh Sleep Quality Index among participants with suboptimal glycemic control [46].
Ethnicity plays a significant role in determining the risk of developing T2D during childhood and adolescence. Epidemiological data consistently show that youth-onset T2D is most prevalent among Native American, Canadian First Nation, Indigenous Australian, Black, Hispanic, East and South Asian, Middle Eastern, and Pacific Islander populations. The elevated prevalence observed in these groups reflects a complex interplay of genetic, environmental, and lifestyle factors. In many of these populations, high obesity rates substantially contribute to T2D risk. Interestingly, East Asian children present an exception to this trend, as they exhibit relatively low obesity prevalence with a disproportionately high incidence of T2D. This pattern is likely attributable to a genetic predisposition to β-cell dysfunction, resulting in inadequate insulin secretion even in the context of minimal adiposity [47].
The Progress in Diabetes Genetics in Youth (ProDiGY) Consortium conducted the first genome-wide association study focused on T2D in children and adolescents, aiming to identify genetic variants associated with early-onset disease. The study identified several risk loci, including rs10992863 in PHF2, rs7903146 in TCF7L2, rs72982988 near MC4R, rs200893788 in CDC123, rs2237892 in KCNQ1, rs937589119 in IGF2BP2, rs113748381 in SLC16A11, and a locus in CPEB2, that influence insulin secretion and glucose metabolism. Whereas the majority of loci identified in youth-onset T2D overlap with those described in adult-onset disease, PHF2 (rs10992863) and CPEB2 appear to be specific to the early-onset form [48].
Furthermore, the N-acetyltransferase 2 (NAT2) A803 allele appears to predispose children to altered glucose tolerance by impairing pancreatic β-cell insulin secretion, as evidenced by a study involving 748 obese children and adolescents, revealing a higher prevalence of both impaired glucose tolerance (IGT) and elevated fasting plasma glucose among individuals carrying this allele, suggesting a potential genetic contribution to metabolic dysregulation in pediatric obesity [49].
In addition to lifestyle and genetic factors, certain medications are also known to increase the risk of early-onset T2D. In recent years, the growing use of antidepressants in children and adolescents has raised concerns about potential metabolic effects. A retrospective cohort study on Medicaid claims data assessing T2D risk among youth taking various classes of antidepressants, showed that long-term use of antidepressants—particularly serotonin-norepinephrine reuptake inhibitors (SNRIs) and tricyclic antidepressants (TCAs)—was associated with a higher risk of developing T2D, especially with prolonged exposure. Long-term exposure to SNRIs and TCAs has been associated with weight gain, impaired insulin sensitivity, and disruption of glucose homeostasis, likely mediated through serotonergic, noradrenergic, and histaminergic pathways that regulate appetite and energy balance [50]. Among other drugs, atypical antipsychotic agents (including aripiprazole, risperidone, olanzapine) are well known risk factors for weight gain and the early onset of T2D. Antipsychotic medications primarily exert their therapeutic effects through antagonism of dopamine D2 receptors. However, many also exhibit affinity for other neurotransmitter systems, including serotonergic (e.g., 5-HT2A), histaminergic (e.g., H1), and adrenergic (e.g., α1) receptors. These systems play key roles in the metabolic regulation and insulin secretion, and their modulation may underlie the metabolic side effects commonly associated with antipsychotic treatment [51].
4. Screening and Diagnosis
Youth-onset T2D presents as a more aggressive and rapidly progressive disease than its adult-onset counterpart [7]. Consequently, early screening and timely diagnosis are essential to prevent both acute and long-term complications.
According to the American Diabetes Association (ADA) guidelines, diabetes screening is recommended for all children aged ≥10 years who have BMI >85th percentile for age and sex and with at least one additional risk factor, as outlined in Table 2 [1].

Table 2.
High-risk groups for youth-onset type 2 diabetes.
The methods currently employed for the screening and diagnosis of T2D in pediatric populations include measurement of HbA1c, fasting plasma glucose (FPG), and plasma glucose concentrations obtained during a 2-h oral glucose tolerance test (OGTT). The results of the tests identify different metabolic conditions based on the degree of glycemic dysregulation [52].
Updated diagnostic criteria of diabetes, according to ADA Guidelines, are summarized in Table 3. A diagnosis of diabetes in pediatric and adolescent populations is established when any of the standard diagnostic criteria are fulfilled on two separate occasions. Additionally, the presence of a random plasma glucose concentration ≥ 200 mg/dL (≥11.1 mmol/L) in conjunction with classic symptoms of hyperglycemia—such as polyuria, polydipsia, or unexplained weight loss—also constitutes diagnostic evidence of diabetes.

Table 3.
Diagnostic criteria of diabetes and prediabetes.
Alternatively, investigations may reveal a state of impaired glucose regulation called prediabetes, which is characterized by abnormalities in carbohydrate metabolism and is considered a potential intermediary stage in the progression to T2D. As defined by the ADA Guidelines, prediabetes is identified based on specific glycemic thresholds indicative of impairment in glucose homeostasis that do not fulfill the diagnostic criteria for diabetes.
The optimal screening method for diabetes in obese youth remains controversial. Alternative glycemic markers, such as glycated albumin, 1,5-anhydroglucitol, and fructosamine, have been proposed as potential tools for T2D screening. However, current evidence has shown their effectiveness in detecting prediabetes is limited compared to traditional markers [53].
HbA1c, the end product of a glycosylation process involving the covalent binding between glucose and the N-terminal valine of the hemoglobin β chain, reflects the concentration of blood glucose over the preceding 8 to 12 weeks. The ADA guidelines highlight several advantages of HbA1c as a diagnostic marker of diabetes, including its convenience, as fasting is not required, and its lower intra-individual variability compared to other markers. A position article evaluated the use of HbA1c as a diagnostic tool for T2D in developing countries, focusing on its lower day-to-day variability as a key advantage. However, the authors also acknowledged several limitations, including reduced sensitivity and limited accessibility in certain regions. Across many low- and middle-income settings, limited access to standardized HbA1c assays constrains its diagnostic use; consequently, fasting plasma glucose remains the most widely implemented test. Additionally, HbA1c values may be underestimated in individuals with hematologic disorders such as sickle cell traits or X-linked glucose-6-phosphate dehydrogenase mutations [54].
An observational study conducted by Ehehalt et al. on a population of German overweight and obese children and adolescents, found that HbA1c is more reproducible and convenient as initial screening test for diabetes in high-risk pediatric populations due to its superior sensitivity, specificity, and practicality compared to OGTT. However, this study also highlighted that HbA1c diagnostic accuracy for prediabetes is limited, necessitating complementary tests [55].
The optimal HbA1c threshold for the early detection of T2D in youth remains a topic of ongoing investigation and debate. Recent evidence suggests that the risk of progression to T2D is approximately 4% among individuals with a baseline HbA1c of 5.7–5.9%, and increases to 8% in those with levels ranging from 6.0% to 6.4% [56]. Several studies have raised concerns about the sensitivity of the current diagnostic threshold of HbA1c of 6.5%, indicating that it may be less effective for early detection in pediatric populations. In a retrospective, single-center study analyzing data from 236 overweight or obese children and adolescents aged 4–17 years, a HbA1c level of 6.5% demonstrated a sensitivity of 87.2% and a specificity of 98.5% for detecting T2D. The optimal HbA1c cutoff identified was >6.2%, which yielded a sensitivity of 94.7% and specificity of 95.5% [57]. On the other hand, another study reported that lowering the HbA1c threshold to detect prediabetes resulted in a high rate of false positives, reducing its specificity and clinical utility. For HbA1c values between 6.0–6.5% (42 and 48 mmol/mol), additional confirmatory testing is necessary to accurately diagnose diabetes [55].
OGTT is a dynamic test that evaluates plasma glucose level 120 min after oral glucose intake and it is particularly useful for evaluating glucose tolerance. OGTT seems to be more sensitive than FPG in detecting early glucose dysregulation and it is the preferred diagnostic test for screening high-risk children when HbA1c and FPG are inconclusive. However, the requirement for fasting and multiple blood draws presents practical challenges in the pediatric population.
Recent studies have proposed novel approaches to interpreting OGTT results for the early diagnosis of T2D in youth. Emerging evidence suggests that evaluating 1-h plasma glucose concentration during the OGTT may offer a reliable alternative to the traditional 2-h measurement in identifying early-stage T2D [58]. Ravà et al. investigated the utility of 1-h glucose levels as a predictor of impaired glucose tolerance and cardiometabolic risk in children with obesity. By analyzing the diagnostic accuracy of various thresholds, they proposed that a cutoff of 155 mg/dL (8.6 mmol/L) could represent a practical and meaningful marker for early T2D detection in the pediatric population [59].
Given that type 1 diabetes (T1D) remains the most prevalent form of diabetes in youth, a comprehensive diagnostic evaluation for T2D should include the assessment of pancreatic autoantibodies, such as glutamic acid decarboxylase antibody (GAD-65), insulinoma-associated antigen-2 antibody (IA-2), zinc transporter 8 antibody (ZnT8), and insulin autoantibody (IAA), to reliably exclude autoimmune diabetes [60,61]. The widespread prevalence of obesity across the general pediatric population has led to increasing overlap in phenotypic features between T1D and T2D. Notably, excess adiposity is no longer a distinctive feature of T2D alone, as a significant proportion of youth with T1D now also present with overweight or obesity at onset. The Treatment Options for type 2 Diabetes in Adolescents and Youth (TODAY) study reported that approximately 12% of individuals who initially met diagnostic criteria for T2D, tested positive for GAD-65 and/or IA-2 antibodies, suggesting underlying autoimmune β-cell dysfunction despite the clinical phenotype being consistent with T2D [62].
5. Clinical Presentation
The clinical course of T2D diagnosed during adolescence is more aggressive compared to adult-onset cases [61]. Data from the TODAY study have demonstrated that youth-onset T2D progresses more rapidly, with a faster decline in β-cell function [62].
The clinical phenotype of children and adolescents diagnosed with T2D is typically characterized by overweight, defined by a BMI at or above the 85th percentile for sex and age, or obesity, defined as a BMI at or above the 95th percentile for age and sex, in accordance with the Centers for Disease Control and Prevention (CDC) growth charts. In addition, many affected individuals exhibit central adiposity, as indicated by a waist circumference exceeding the 90th percentile for age and sex. This anthropometric profile is frequently accompanied by clinical and biochemical markers of insulin resistance including acanthosis nigricans, dyslipidemia, and arterial hypertension.
The clinical presentation of T2D at onset in children and adolescents varies widely, from asymptomatic to severe conditions including diabetic ketoacidosis (DKA) and hyperglycemic hyperosmolar syndrome (HHS).
DKA is an acute metabolic complication of diabetes characterized by hyperglycemia (blood glucose > 200 mg/dL or 11 mmol/L), venous pH < 7.3 or serum bicarbonate <18 mmol/L, ketonemia (blood ß-hydroxybutyrate ≥ 3 mmol/L) or moderate or large ketonuria. Ketosis-prone diabetes (KPD) is a form of diabetes that exhibits features of both T1D and T2D. Subjects typically present with DKA at onset and phenotype of obesity-associated T2D, with exact underlying mechanisms remaining under investigation. The Aβ classification system categorizes KPD based on the presence or absence of β-cell autoantibodies (A+ or A−) and β-cell functional reserve (β+ or β−). Pathophysiologically, A−β+ KPD appears to reflect transient β-cell secretory failure on a background of insulin resistance, with ketoacidosis driven by impaired ketone oxidation and altered substrate handling rather than excess ketogenesis [63]. Data from the Rare and Atypical Diabetes Network (RADIANT) showed that 45% of children diagnosed with T2D presenting with DKA met the criteria for the A−β+ KPD, highlighting the importance of considering this diagnosis in youth as well as in adults [64].
Another possible clinical presentation of T2D is euglycemic DKA (EDKA), a rare but serious metabolic complication characterized by significant ketoacidosis with blood glucose levels < 250 mg/dL. While traditionally associated with T1D, euglycemic DKA has been increasingly reported in individuals with T2D. Vomiting, abdominal pain, fatigue could represent the clinical presentation of this condition so EDKA could be mistaken with gastroenteritis or infection. EDKA could be caused by the use of SGLT2 inhibitors, prolonged fasting or eating disorders, inadequate insulin treatment [65].
HHS is a serious and potentially fatal complication of diabetes characterized by extreme elevations in serum glucose concentrations (>600 mg/dL) and hyperosmolality without significant ketosis and acidosis. The incidence of HHS has risen in parallel with the increasing prevalence of childhood obesity and T2D, with reports indicating that up to 2% of children may present with HHS at diagnosis [66]. This severe condition often presents with neurological manifestations, severe dehydration, with sepsis or infection often acting as a trigger. Identified risk factors that may lead to HHS are delayed diagnosis of T2D and poor access to care. In children and adolescents presenting with HHS, aggressive fluid resuscitation is often necessary to prevent hemodynamic instability and circulatory collapse. During rehydration serum sodium concentrations should be closely monitored and progressive decline in serum glucose is expected [67].
6. Complications
Long-term complications of T2D may occur earlier compared to T1D (Figure 1) [62]. Furthermore, early-onset T2D is associated with a more severe phenotype compared to adult counterpart, as suggested by data from an Australian cohort of 193 youth with T2D, where nine individuals had already developed at least one diabetes-related complication at the time of diagnosis [68].
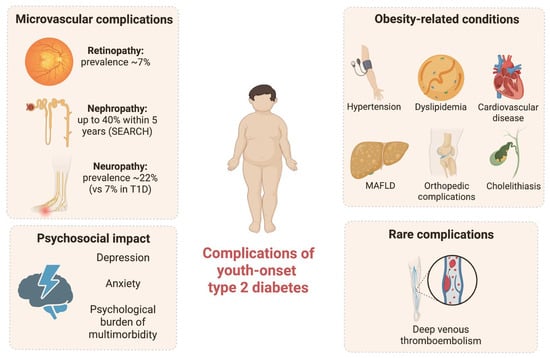
Figure 1.
Complications of youth-onset type 2 diabetes. Summary of microvascular, obesity-related, psychosocial, and rare complications associated with pediatric type 2 diabetes.
Diabetic nephropathy, characterized by persistent albuminuria and declining renal function, is a significant concern in youth with T2D. Different researchers have showed evidence of early kidney damage in youth with T2D. The SEARCH study has shown that up to 40% of adolescents with T2D develop microalbuminuria within five years after diagnosis. Additionally, albuminuria progression seems to be more common in T2D versus T1D [22]. The TODAY study is a landmark clinical trial that followed adolescents with T2D over several years. By the third year, about 6% of participants had developed microalbuminuria, and the prevalence increased over time [62].
Diabetic retinopathy is a serious microvascular complication of T2D, and although traditionally considered a late-onset complication, it has increasingly been reported in children and adolescents with T2D. A recent systematic review and meta-analysis of 27 observational studies including children and adolescents with T2D showed that diabetic retinopathy was present among 6.99% of participants, with prevalence increasing significantly with longer diabetes duration [69].
Diabetic peripheral neuropathy is another potential complication of T2D in pediatric age. A study by Jaiswal et al. found that its prevalence was 7% in youth with T1D and 22% in youth with T2D [70].
Besides its association with T2D, obesity is a chronic condition that may lead to several complications. Arterial hypertension, dyslipidemia, cardiovascular disease, metabolic dysfunction-associated fatty liver disease (MAFLD), genu valgum, flatfoot, and cholelithiasis are some of the obesity-associated comorbidities in youth.
Sporadic cases of deep venous thromboembolism in the context of T2D have also been documented in the pediatric population. A reported case involved a 15-year-old male who presented with swelling and pain in the left calf and was ultimately diagnosed with extensive deep venous thromboembolism. A concurrent diagnosis of T2D was made, supporting the hypothesis of a potential interplay between metabolic dysregulation and an underlying genetic predisposition to thrombosis [71].
Finally, the association between T2D and other obesity-related conditions in young age may also represent a significant psychological burden. Data from the Pediatric Diabetes Consortium T1D and T2D registries confirmed that youth with T2D had higher rates of depressive symptoms and anxiety compared to those with T1D [72].
7. Management
Given the known association of youth-onset T2D with both microvascular and macrovascular complications within the first years of disease, maintaining optimal glycemic control in this population is crucial [73]. Management of T2D needs a comprehensive approach that involves education, lifestyle modifications, regular physical activity, and, when indicated, intensive glucose monitoring and pharmacologic treatment [7] (Figure 2). However, in pediatric populations, achieving therapeutic goals can be challenging due to the limited availability of approved treatment options and the complexities associated with growth and development [73].
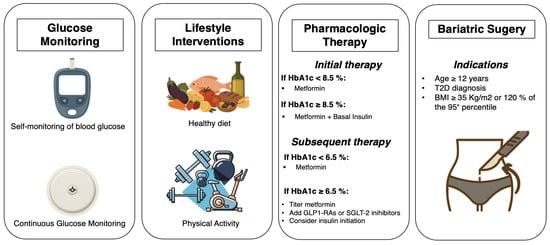
Figure 2.
Graphical summary of management strategies for youth-onset type 2 diabetes.
7.1. Lifestyle
Lifestyle modification represents the first-line intervention in individuals with prediabetes, aiming to delay or prevent the progression to T2D, and a cornerstone of therapeutic management in youth with established T2D, in association to pharmacologic treatment.
Several interventional studies have demonstrated the benefits of structured lifestyle programs in youth at risk for, or with, established T2D. Large-scale community-based prevention trials reported reductions in obesity-related outcomes [74], whereas smaller randomized programs in high-risk adolescents consistently improved physical activity, insulin sensitivity, health-related quality of life, and body composition, even when effects on BMI or HbA1c were modest [75,76].
However, not all lifestyle interventions have proven effective. The Feel4Diabetes program did not significantly increase physical activity, highlighting barriers such as socioeconomic disadvantage, limited parental education, and lack of time [77,78,79].
Data from the TODAY study underscore that sustained adherence to dietary and physical-activity recommendations is associated with improved glycemic control; nevertheless, overall adherence remains challenging [80,81]. Psychosocial factors, including depression, stressful life events, and limited family or peer support, are significant obstacles to long-term behavioral change [82,83,84].
Nutritional strategies remain central to both prevention and treatment. Observational and interventional evidence indicates that meal timing, glycemic index, and breakfast consumption influence insulin sensitivity and weight regulation [85,86,87,88]. Emerging studies further suggest potential benefits of adjunctive dietary supplements, such as a polysaccharide macromolecule complex, in delaying T2D progression, although the evidence remains preliminary [89]. Vitamin D deficiency is frequently reported in youth with obesity and T2D and has been associated with reduced insulin sensitivity and adverse metabolic outcomes in observational studies. Some interventional trials suggest that vitamin D supplementation may improve insulin resistance, yet evidence remains inconsistent and insufficient to establish a protective role in preventing or delaying T2D onset [90].
Physical activity is a critical determinant of metabolic health. A combination of aerobic and resistance training appears most effective in reducing insulin resistance, while both endurance and high-intensity interval training have been shown to improve glycemic control, body composition, and cardiorespiratory fitness [91,92,93]. Additional interventional studies have demonstrated favorable effects on lipid profiles, hepatic fat, neurocognitive function, and cardiovascular parameters [94,95,96,97,98].
7.2. Glucose Monitoring
In recent years, technological advancements have substantially transformed diabetes management, offering increasingly sophisticated tools for monitoring and treatment. Among these, continuous glucose monitoring (CGM) systems have represented a major step forward, particularly in pediatric T1D, where they are now widely considered as the standard of care. By assessing glucose concentrations in the interstitial fluid, CGM devices allow real-time monitoring of glycemic levels, providing users and healthcare providers with valuable information to support diabetes management [99].
Although CGM systems have been extensively validated in T1D, recent evidence has also highlighted their potential role in T2D, including in pediatric populations. Glucose sensors are being explored both as diagnostic tools, offering a less invasive alternative to the OGTT to evaluate response to glucose intake, and as monitoring instruments in people with established T2D, including individuals on insulin as well as those receiving non-insulin hypoglycemic therapies.
A recent study involving 39 overweight or obese youth with prediabetes evaluated the performance of CGM as a screening tool for T2D during an OGTT, comparing its results with conventional plasma glucose measurements. While CGM was generally well tolerated, its clinical applicability was limited by challenges in accurately identifying the timing of glucose ingestion and by the lack of standardized and recognized dysglycemia thresholds [100].
The benefits of CGM use in youth with established T2D are still under debate. A study exploring the feasibility and outcomes of CGM use in adolescents with T2D reported improvements in HbA1c after three months, along with increased patient satisfaction [101]. These beneficial effects were further supported by Chesser et al., who demonstrated enhanced Pediatric Quality of Life Inventory (PedsQL) scores in a cohort of adolescents and young adults with uncontrolled T2D (HbA1c > 7%) [102].
In contrast, Manfredo et al. found that CGM use in youth with T2D did not yield significant improvements in short- or long-term glycemic control, although it did contribute to promoting behavioral improvements [103].
7.3. Metformin
Metformin, combined with lifestyle modifications, is the first line treatment in pediatric subjects with T2D and HbA1c values < 8.5% [7]. Metformin belongs to a class of oral hypoglycemic agents known as biguanides, and is approved by FDA for pediatric use in children aged 10 years and older [104]. Metformin hypoglycemic effect relies on its ability to inhibit hepatic gluconeogenesis and lipogenesis while increasing fatty acid oxidation. It also acts by increasing glucose uptake and fatty acid oxidation in skeletal muscle and adipose tissue, decreasing glucose intestinal absorption and increasing its elimination, facilitating glucose utilization by intestinal cells and promoting GLP-1 secretion [104].
It can be administered with a starting daily dose of 500 mg that can be increased up to the maximum tolerated dose and not exceeding 2000 mg per day. Potential side effects of biguanides are transient abdominal pain, diarrhoea, and nausea, while their use is contraindicated in subjects with diabetic ketoacidosis, eGFR < 30 mL/min, cardiac insufficiency, respiratory insufficiency and in individuals who receive radiographic contrast materials [7].
Metformin is the most commonly prescribed oral medication for the treatment of pediatric T2D, as highlighted by the SEARCH study, which reported that 64.9% of enrolled youth with T2D received metformin monotherapy, while 70.4% were treated with metformin in combination with insulin [105]. Kelsey et al., examining clinical characteristics in a large cohort of adolescents with recent-onset T2D screened for the TODAY study, demonstrated that metformin therapy, when combined with proper diabetes education, improved short-term glycemic control and reduced cardiovascular risk factors [106].
Despite being the first-line therapy and one of the few medications available in youth-onset T2D, a significant limitation of metformin therapy is the high rate of therapeutic failure [107]. Bacha et al., analyzing data from the Pediatric Diabetes Consortium T2D Registry involving 276 youths with T2D, identified two predictive factors associated with successful long-term glycemic control using metformin alone: lower baseline HbA1c values and shorter duration of diabetes at the time of diagnosis [108]. These findings were further supported by an analysis of data from the TODAY study, which identified the HbA1c cutoff of 6.3% during metformin therapy as a useful indicator of high risk for therapeutic failure [109]. In this context, the advantage of metformin as a widely available and low-cost therapy must be balanced against its limited long-term durability, with therapeutic failure remaining a frequent outcome in adolescents.
Moreover, adherence to oral metformin regimens remains challenging for many adolescents. Venditti et al. identified key barriers to optimal adherence, including forgetfulness, inconsistent meal timing, irregular sleep patterns, and daily schedule disruptions. Conversely, the presence of strong family support and consistent daily routines emerged as effective strategies to enhance adherence and improve treatment outcomes among adolescents with T2D [110].
7.4. GLP-1 Receptor Agonists (GLP-1 RAs)
Recently, GLP-1 receptor agonists (GLP1-RAs) have emerged as a valuable therapeutic option for managing obesity and T2D in pediatric populations. In line with the latest ISPAD guidelines, these medications are recommended for adolescents unable to achieve or maintain HbA1c levels below 6.5% with first-line therapies [7]. GLP1-RAs facilitate weight loss and support β-cell function, thereby contributing to better regulation of blood glucose levels. Apart from pancreatic β-cells, GLP1-RAs exert their effects on multiple organs such as brain, heart, liver, stomach, and intestine with the result of favoring appetite suppression, delaying gastric emptying, improving heart function, and decreasing liver fat content [111]. FDA-approved molecules belonging to this class are liraglutide, semaglutide, exenatide, and dulaglutide, that can be administered in children aged 10 years and older. GLP1-RAs may be responsible for gastrointestinal side effects, such as nausea, vomiting and diarrhea and, less frequently, may cause dizziness, headache and dyspepsia [7]. Although GLP-1 receptor agonists offer superior efficacy in terms of weight reduction and glycemic control, their high cost and limited reimbursement in several healthcare systems currently restrict their widespread applicability in pediatric populations.
Miller et al. analyzed clinical and demographic features of a cohort of 11,380 preadolescents and adolescents treated with GLP-1 RAs, showing that the majority had a previous T2D diagnosis, a history of metformin use, or a BMI exceeding the 95th percentile [112].
The effects of GLP-1 RAs on glucose control and body weight were evaluated in a meta-analysis led by Dai et al., which included 14 randomized controlled trials involving 1262 adolescents with overweight/obesity and/or T2D. The results showed significant improvements in HbA1c levels, fasting glucose and body weight. Interestingly, liraglutide exhibited superior efficacy in terms of glucose control, while exenatide was more effective in promoting weight loss [113]. Another meta-analysis analyzing five studies involving a total of 415 children with T2D reached similar conclusions, also reporting negligible gastrointestinal side effects reported [114].
Additionally, a Korean study investigated the efficacy of once-weekly dulaglutide in five pediatric subjects with T2D, four of whom had previously been treated with metformin. After a 12-month follow-up, dulaglutide was found to significantly improve HbA1c levels [115].
7.5. Sodium-Glucose Cotransporter 2 (SGLT2) Inhibitors
SGLT2 inhibitors act by promoting the excretion of glucose through the renal tubules, thereby lowering blood glucose levels independently of insulin secretion. According to the most recent ISPAD guidelines, their use should be considered when the therapeutic goal of maintaining HbA1c below 6.5% is not achieved, either as monotherapy or in combination with GLP-1 RAs [7,116].
Dapagliflozin, an SGLT2 inhibitor indicated for the treatment of diabetes, cardiac insufficiency and kidney disease in adults, has recently been investigated in pediatric populations and is currently approved by FDA and EMA for use in children aged 10 years and older [7]. A systematic review by Grube et al., including five studies on the use oral dapagliflozin in children aged 0 to 17 years, suggested good safety and efficacy profiles for the drug [117]. A clinical trial involving 24 adolescents with T2D aged 10 to 17, treated with daily doses of 2.5, 5 or 10 mg of dapagliflozin, showed a dose-dependent increase in urinary glucose excretion and a reduction in mean fasting plasma glucose across all treatment groups, with only six subjects experiencing mild adverse effects [118]. The T2NOW clinical trial, which compared dapagliflozin with saxagliptin in youths aged 10 to 17 with T2D, found superior glycemic outcomes with dapagliflozin [119]. In the follow-up extension of the trial, 52 weeks of dapagliflozin therapy did not adversely affect growth, pubertal development, bone markers, or overall maturation up to one year after treatment discontinuation [120]. Nonetheless, available pediatric studies are short in duration, and long-term safety and durability of metabolic benefits remain to be established. In addition, cost-related barriers may limit real-world uptake, particularly outside well-resourced healthcare systems.
Canagliflozin, another SGLT2 inhibitor, was evaluated in a clinical trial involving a pediatric population randomized to receive either 100 mg or 300 mg daily. After 14 days of treatment, both doses showed effectiveness in reducing the mean 24-h renal threshold for glucose and increased urinary glucose excretion, while maintaining good tolerability and acceptability [121].
7.6. Dipeptidyl Peptidase-4 (DPP-4) Inhibitors
By inhibiting the degradation of incretin hormones, DPP-4 inhibitors enhance glucose-dependent insulin secretion and have become a valuable therapeutic option in adults with T2D. More recently, their potential applicability in the pediatric population has begun to receive increasing attention.
Tamborlane et al., in a randomized controlled trial involving 39 adolescents with T2D aged 10 to 18 years, compared linagliptin to placebo at once-daily doses of 1 mg and 5 mg over a 12-week period. The study reported dose-dependent improvements in HbA1c and fasting plasma glucose, with no adverse effects observed [122]. Similarly, sitagliptin, evaluated at single doses of 50, 100, or 200 mg in a trial including 35 adolescents with T2D, showed good overall tolerability [123]. It should be noted, however, that pediatric-specific data on DPP-4 inhibitors remain extremely limited, and most efficacy and safety considerations are extrapolated from adult studies.
7.7. Insulin Therapy
According to ISPAD guidelines, basal insulin is recommended alongside metformin as initial therapy for youth with T2D who present with an HbA1c ≥ 8.5% at diagnosis. Additionally, the initiation of basal insulin—and, if needed, short-acting insulin—is suggested when lifestyle modifications, metformin, SGLT-2 inhibitors, and GLP1-RAs fail to achieve HbA1c levels < 6.5%. In cases of diabetic ketoacidosis or hyperglycemic hyperosmolar syndrome at presentation, intravenous insulin followed by multiple daily injections of long- and short-acting insulin is indicated [7].
A randomized controlled trial evaluated whether early combination therapy with glargine and metformin, compared to metformin monotherapy, could preserve β-cells destruction—a key aspect of T2D pathophysiology. The study, which enrolled 91 overweight or obese youths with impaired glucose tolerance or recently diagnosed T2D, showed that neither approach arrested progressive β-cell damage [124].
Concerns regarding hypoglycemia often arise with insulin use in T2D. Shahid et al. evaluated the real-world impact of hypoglycemia in a cohort of 22 adolescents with T2D receiving insulin therapy. Over a 3-month follow-up period, nine episodes of hypoglycemia were recorded, of which only five were symptomatic. Interestingly, a positive correlation was observed between the frequency of hypoglycemia and lower BMI [125].
Given the substantial advances in diabetes technology, closed-loop insulin delivery systems have already demonstrated clinical benefits in adults with T2D [126]. However, further research is needed to assess their efficacy and safety in pediatric populations with T2D.
7.8. Bariatric Surgery
Given the evidence that bariatric surgery can lead to improvement or even remission of T2D in adults, its potential application in pediatric populations has become a topic of interest within the scientific community [127]. Bariatric surgery is currently recommended for youth with T2D and a BMI ≥ 35 kg/m2 or ≥120% of the 95th percentile, who are over 12 years of age [7]. Even though data on long-term effects of bariatric surgery remain limited, researchers have also hypothesized a possible role for bariatric surgery also in T2D prevention [128].
Inge et al., in a clinical trial comparing medical therapy and bariatric surgery in severely obese adolescents with T2D, found that surgery was more effective in improving glycemic control, promoting weight loss, and reducing comorbidities. However, 23% of participants undergoing surgery required additional surgical interventions during the two-year follow-up period [129].
A study focusing on laparoscopic sleeve gastrectomy in 64 pediatric patients with T2D reported significant reductions in HbA1c and random blood glucose levels following the procedure [130]. Similarly, Carbajo et al. observed a 100% remission rate after five years in a cohort of obese adolescents who had undergone one-anastomosis gastric bypass. Notably, only one case of iron deficiency anemia was reported, with no instances of malnutrition or growth impairment—supporting the relative safety of the procedure in selected patients [131].
8. Conclusions
Youth-onset T2D is rising worldwide, with evidence from large studies showing a rapid increase over the past two decades. Early-onset disease carries a more aggressive course, with higher rates of complications such as nephropathy, retinopathy, and MAFLD, and is strongly linked to obesity, minority ethnicity, and socioeconomic disadvantage. These findings underline the urgent need for prevention, timely diagnosis, and effective interventions to reduce the long-term burden.
Author Contributions
Conceptualization, B.B. and S.P.; writing—original draft preparation, A.T., S.S., E.I. and M.P.; writing—review and editing, B.B., S.P. and M.V.; supervision, F.L. and G.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
B.B. reports grants from Movi SpA and Abbott. F.L. received speaker and consultant honoraria from Sanofi and speaking honoraria from Movi SpA. S.P. received speaking honoraria from Movi SpA. No other potential conflicts of interest relevant to this article were reported.
References
- American Diabetes Association Professional Practice Committee. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes-2025. Diabetes Care 2025, 48 (Suppl. S1), S27–S49. [Google Scholar] [CrossRef]
- Mayer-Davis, E.J.; Lawrence, J.M.; Dabelea, D.; Divers, J.; Isom, S.; Dolan, L.; Imperatore, G.; Linder, B.; Marcovina, S.; Pettitt, D.J.; et al. Incidence Trends of Type 1 and Type 2 Diabetes among Youths, 2002–2012. N. Engl. J. Med. 2017, 376, 1419–1429. [Google Scholar] [CrossRef]
- Dabelea, D.; Mayer-Davis, E.J.; Saydah, S.; Imperatore, G.; Linder, B.; Divers, J.; Bell, R.; Badaru, A.; Talton, J.W.; Crume, T.; et al. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA 2014, 311, 1778–1786. [Google Scholar] [CrossRef] [PubMed]
- Reinehr, T. Long-term effects of adolescent obesity: Time to act. Nat. Rev. Endocrinol. 2018, 14, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Bjerregaard, L.G.; Jensen, B.W.; Ängquist, L.; Osler, M.; Sørensen, T.I.A.; Baker, J.L. Change in Overweight from Childhood to Early Adulthood and Risk of Type 2 Diabetes. N. Engl. J. Med. 2018, 378, 1302–1312. [Google Scholar] [CrossRef]
- Arslanian, S.A. Type 2 diabetes mellitus in children: Pathophysiology and risk factors. J. Pediatr. Endocrinol. Metab. 2000, 13 (Suppl. S6), 1385–1394. [Google Scholar] [CrossRef]
- Shah, A.S.; Barrientos-Pérez, M.; Chang, N.; Fu, J.F.; Hannon, T.S.; Kelsey, M.; Peña, A.S.; Pinhas-Hamiel, O.; Urakami, T.; Wicklow, B.; et al. ISPAD Clinical Practice Consensus Guidelines 2024: Type 2 Diabetes in Children and Adolescents. Horm. Res. Paediatr. 2024, 97, 555–583. [Google Scholar] [CrossRef] [PubMed]
- Tinajero, M.G.; Malik, V.S. An Update on the Epidemiology of Type 2 Diabetes: A Global Perspective. Endocrinol. Metab. Clin. N. Am. 2021, 50, 337–355. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Park, H.S.; Huh, B.W.; Seo, S.H.; Seo, D.H.; Ahn, S.H.; Hong, S.; Suh, Y.J.; Kim, S.H. Prevalence and risk of diabetic complications in young-onset versus late-onset type 2 diabetes mellitus. Diabetes Metab. 2022, 48, 101389. [Google Scholar] [CrossRef]
- Bacha, F.; Hannon, T.S.; Tosur, M.; Pike, J.M.; Butler, A.; Tommerdahl, K.L.; Zeitler, P.S. Pathophysiology and Treatment of Prediabetes and Type 2 Diabetes in Youth. Diabetes Care 2024, 47, 2038–2049. [Google Scholar] [CrossRef]
- Valaiyapathi, B.; Gower, B.; Ashraf, A.P. Pathophysiology of Type 2 Diabetes in Children and Adolescents. Curr. Diabetes Rev. 2020, 16, 220–229. [Google Scholar]
- Bombaci, B.; Torre, A.; Longo, A.; Pecoraro, M.; Papa, M.; Sorrenti, L.; La Rocca, M.; Lombardo, F.; Salzano, G. Psychological and Clinical Challenges in the Management of Type 1 Diabetes during Adolescence: A Narrative Review. Children 2024, 11, 1085. [Google Scholar] [CrossRef]
- Kelsey, M.M.; Zeitler, P.S. Insulin Resistance of Puberty. Curr. Diabetes Rep. 2016, 16, 64. [Google Scholar] [CrossRef]
- Robertson, R.P.; Harmon, J.; Tran, P.O.T.; Poitout, V. Beta-cell glucose toxicity, lipotoxicity, and chronic oxidative stress in type 2 diabetes. Diabetes 2004, 53 (Suppl. S1), S119–S124. [Google Scholar] [CrossRef]
- Zhang, K.; Kan, C.; Han, F.; Zhang, J.; Ding, C.; Guo, Z.; Huang, N.; Zhang, Y.; Hou, N.; Sun, X. Global, Regional, and National Epidemiology of Diabetes in Children From 1990 to 2019. JAMA Pediatr. 2023, 177, 837–846. [Google Scholar] [CrossRef]
- Imperatore, G.; Boyle, J.P.; Thompson, T.J.; Case, D.; Dabelea, D.; Hamman, R.F.; Lawrence, J.M.; Liese, A.D.; Liu, L.L.; Mayer-Davis, E.J.; et al. Projections of type 1 and type 2 diabetes burden in the U.S. population aged <20 years through 2050: Dynamic modeling of incidence, mortality, and population growth. Diabetes Care 2012, 35, 2515–2520. [Google Scholar] [CrossRef]
- Wu, H.; Patterson, C.C.; Zhang, X.; Ghani, R.B.A.; Magliano, D.J.; Boyko, E.J.; Ogle, G.D.; Luk, A.O. Worldwide estimates of incidence of type 2 diabetes in children and adolescents in 2021. Diabetes Res. Clin. Pract. 2022, 185, 109785. [Google Scholar] [CrossRef] [PubMed]
- Divers, J.; Mayer-Davis, E.J.; Lawrence, J.M.; Isom, S.; Dabelea, D.; Dolan, L.; Imperatore, G.; Marcovina, S.; Pettitt, D.J.; Pihoker, C.; et al. Trends in Incidence of Type 1 and Type 2 Diabetes Among Youths—Selected Counties and Indian Reservations, United States, 2002–2015. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Galler, A.; Stange, T.; Müller, G.; Näke, A.; Vogel, C.; Kapellen, T.; Bartelt, H.; Kunath, H.; Koch, R.; Kiess, W.; et al. Incidence of childhood diabetes in children aged less than 15 years and its clinical and metabolic characteristics at the time of diagnosis: Data from the Childhood Diabetes Registry of Saxony, Germany. Horm. Res. Paediatr. 2010, 74, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Srivastava, S.; Mishra, P.S.; Mooss, E.T.K. Prevalence of pre-diabetes/type 2 diabetes among adolescents (10–19 years) and its association with different measures of overweight/obesity in India: A gendered perspective. BMC Endocr. Disord. 2021, 21, 146. [Google Scholar] [CrossRef]
- Lawrence, J.M.; Divers, J.; Isom, S.; Saydah, S.; Imperatore, G.; Pihoker, C.; Marcovina, S.M.; Mayer-Davis, E.J.; Hamman, R.F.; Dolan, L.; et al. Trends in Prevalence of Type 1 and Type 2 Diabetes in Children and Adolescents in the US, 2001–2017. JAMA 2021, 326, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Hamman, R.F.; Bell, R.A.; Dabelea, D.; D’Agostino, R.B.; Dolan, L.; Imperatore, G.; Lawrence, J.M.; Linder, B.; Marcovina, S.M.; Mayer-Davis, E.J.; et al. The SEARCH for Diabetes in Youth study: Rationale, findings, and future directions. Diabetes Care 2014, 37, 3336–3344. [Google Scholar] [CrossRef]
- Misra, S.; Holman, N.; Barron, E.; Knighton, P.; Warner, J.; Kar, P.; Young, B.; Valabhji, J. Characteristics and care of young people with type 2 diabetes included in the national diabetes audit datasets for England. Diabet. Med. 2023, 40, e14940. [Google Scholar] [CrossRef]
- Copeland, K.C.; Zeitler, P.; Geffner, M.; Guandalini, C.; Higgins, J.; Hirst, K.; Kaufman, F.R.; Linder, B.; Marcovina, S.; McGuigan, P.; et al. Characteristics of adolescents and youth with recent-onset type 2 diabetes: The TODAY cohort at baseline. J. Clin. Endocrinol. Metab. 2011, 96, 159–167. [Google Scholar] [CrossRef]
- Stahl-Pehe, A.; Baechle, C.; Lanzinger, S.; Urschitz, M.S.; Reinauer, C.; Kamrath, C.; Holl, R.W.; Rosenbauer, J. Trends in the incidence of type 1 diabetes and type 2 diabetes in children and adolescents in North Rhine-Westphalia, Germany, from 2002 to 2022. Diabetes Metab. 2024, 50, 101567. [Google Scholar] [CrossRef] [PubMed]
- Molina-Díaz, J.M.; Vargas-Terrez, B.E.; Medina-Bravo, P.G.; Martínez-Ambrosio, A.; Miranda-Lora, A.L.; Klünder-Klünder, M. Prevalence of type 2 diabetes mellitus in the pediatric population of a third-level care hospital in Mexico City in 2013 and 2018. World J. Diabetes 2023, 14, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Dubayee, M.A.; Juraibah, F.A.; Alfaraidi, H.; Alghnam, S.; Aldahash, R.; Attia, N.; Shaikh, A.; Habeb, A.; Jabri, A.; Zaben, A.; et al. Establishing the Saudi pediatric and youth diabetes registry: Initial data and challenges. Sudan. J. Paediatr. 2024, 24, 10–20. [Google Scholar] [CrossRef]
- Abdelhameed, F.; Giuffrida, A.; Thorp, B.; Moorthy, M.K.; Gevers, E.F. Exploring the Surge in Paediatric Type 2 Diabetes in an Inner-City London Centre-A Decade-Long Analysis of Incidence, Outcomes, and Transition. Children 2024, 11, 173. [Google Scholar] [CrossRef]
- Zuckerman Levin, N.; Cohen, M.; Phillip, M.; Tenenbaum, A.; Koren, I.; Tenenbaum-Rakover, Y.; Admoni, O.; Hershkovitz, E.; Haim, A.; Aronovitch, K.M.; et al. Youth-onset type 2 diabetes in Israel: A national cohort. Pediatr. Diabetes 2022, 23, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Sellers, E.A.C.; McLeod, L.; Prior, H.J.; Dragan, R.; Wicklow, B.A.; Ruth, C. Incidence and prevalence of type 2 diabetes in Manitoba children 2009–10 to 2017–18: First Nation versus all other Manitobans. Diabetes Res. Clin. Pract. 2024, 208, 111097. [Google Scholar] [CrossRef]
- Bombaci, B.; Passanisi, S.; Sorrenti, L.; Salzano, G.; Lombardo, F. Examining the associations between COVID-19 infection and pediatric type 1 diabetes. Expert. Rev. Clin. Immunol. 2023, 19, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Magge, S.N.; Wolf, R.M.; Pyle, L.; Brown, E.A.; Benavides, V.C.; Bianco, M.E.; Chao, L.C.; Cymbaluk, A.; Balikcioglu, P.G.; Halpin, K.; et al. The Coronavirus Disease 2019 Pandemic is Associated with a Substantial Rise in Frequency and Severity of Presentation of Youth-Onset Type 2 Diabetes. J. Pediatr. 2022, 251, 51–59.e2. [Google Scholar] [CrossRef] [PubMed]
- Lange, S.J.; Kompaniyets, L.; Freedman, D.S.; Kraus, E.M.; Porter, R.; DNP3; Blanck, H.M.; Goodman, A.B. Longitudinal Trends in Body Mass Index Before and During the COVID-19 Pandemic Among Persons Aged 2–19 Years—United States, 2018–2020. MMWR Morb. Mortal Wkly. Rep. 2021, 70, 1278–1283. [Google Scholar] [CrossRef]
- DeLacey, S.; Arzu, J.; Levin, L.; Ranganna, A.; Swamy, A.; Bianco, M.E. Impact of SARS-CoV2 on youth onset type 2 diabetes new diagnoses and severity. J. Diabetes 2022, 14, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Denzer, C.; Rosenbauer, J.; Klose, D.; Körner, A.; Reinehr, T.; Baechle, C.; Schröder, C.; Wiegand, S.; Holl, R.W.; Prinz, N.; et al. Is COVID-19 to Blame? Trends of Incidence and Sex Ratio in Youth-Onset Type 2 Diabetes in Germany. Diabetes Care 2023, 46, 1379–1387. [Google Scholar] [CrossRef]
- Hani, N.S.; Thomas, I.H. Changes in Pediatric Type 2 Diabetes During the COVID-19 Pandemic. Pediatr. Ann. 2024, 53, e249–e253. [Google Scholar] [CrossRef]
- Abbasi, A.; Juszczyk, D.; van Jaarsveld, C.H.M.; Gulliford, M.C. Body Mass Index and Incident Type 1 and Type 2 Diabetes in Children and Young Adults: A Retrospective Cohort Study. J. Endocr. Soc. 2017, 1, 524–537. [Google Scholar] [CrossRef]
- Nelson, M.C.; Neumark-Stzainer, D.; Hannan, P.J.; Sirard, J.R.; Story, M. Longitudinal and secular trends in physical activity and sedentary behavior during adolescence. Pediatrics 2006, 118, e1627–e1634. [Google Scholar] [CrossRef]
- Verloigne, M.; Van Lippevelde, W.; Maes, L.; Yıldırım, M.; Chinapaw, M.; Manios, Y.; Androutsos, O.; Kovacs, E.; Bringolf-Isler, B.; Brug, J.; et al. Levels of physical activity and sedentary time among 10- to 12-year-old boys and girls across 5 European countries using accelerometers: An observational study within the ENERGY-project. Int. J. Behav. Nutr. Phys. Act. 2012, 9, 34. [Google Scholar] [CrossRef]
- Boyko, E.J.; Fujimoto, W.Y.; Leonetti, D.L.; Newell-Morris, L. Visceral adiposity and risk of type 2 diabetes: A prospective study among Japanese Americans. Diabetes Care 2000, 23, 465–471. [Google Scholar] [CrossRef] [PubMed]
- González, N.; Moreno-Villegas, Z.; González-Bris, A.; Egido, J.; Lorenzo, Ó. Regulation of visceral and epicardial adipose tissue for preventing cardiovascular injuries associated to obesity and diabetes. Cardiovasc. Diabetol. 2017, 16, 44. [Google Scholar] [CrossRef]
- Maffeis, C.; Morandi, A. Body composition and insulin resistance in children. Eur. J. Clin. Nutr. 2018, 72, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Franzago, M.; Fraticelli, F.; Stuppia, L.; Vitacolonna, E. Nutrigenetics, epigenetics and gestational diabetes: Consequences in mother and child. Epigenetics 2019, 14, 215–235. [Google Scholar] [CrossRef] [PubMed]
- Halipchuk, J.; Temple, B.; Dart, A.; Martin, D.; Sellers, E.A.C. Prenatal, Obstetric and Perinatal Factors Associated with the Development of Childhood-Onset Type 2 Diabetes. Can. J. Diabetes 2018, 42, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Darendeliler, F. IUGR: Genetic influences, metabolic problems, environmental associations/triggers, current and future management. Best Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 101260. [Google Scholar] [CrossRef]
- Tang, Y.; Meng, L.; Li, D.; Yang, M.; Zhu, Y.; Li, C.; Jiang, Z.; Yu, P.; Li, Z.; Song, H.; et al. Interaction of sleep quality and sleep duration on glycemic control in patients with type 2 diabetes mellitus. Chin. Med. J. 2014, 127, 3543–3547. [Google Scholar]
- Magliano, D.J.; Boyko, E.J.; IDF Diabetes Atlas 10th Edition Scientific Committee. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- Srinivasan, S.; Chen, L.; Todd, J.; Divers, J.; Gidding, S.; Chernausek, S.; Gubitosi-Klug, R.A.; Kelsey, M.M.; Shah, R.; Black, M.H.; et al. The First Genome-Wide Association Study for Type 2 Diabetes in Youth: The Progress in Diabetes Genetics in Youth (ProDiGY) Consortium. Diabetes 2021, 70, 996–1005. [Google Scholar] [CrossRef]
- Marzuillo, P.; Di Sessa, A.; Umano, G.R.; Nunziata, L.; Cirillo, G.; Perrone, L.; del Giudice, E.M.; Grandone, A. Novel association between the nonsynonymous A803G polymorphism of the N-acetyltransferase 2 gene and impaired glucose homeostasis in obese children and adolescents. Pediatr. Diabetes 2017, 18, 478–484. [Google Scholar] [CrossRef]
- Burcu, M.; Zito, J.M.; Safer, D.J.; Magder, L.S.; dosReis, S.; Shaya, F.T.; Rosenthal, G.L. Association of Antidepressant Medications with Incident Type 2 Diabetes Among Medicaid-Insured Youths. JAMA Pediatr. 2017, 171, 1200–1207. [Google Scholar] [CrossRef]
- Holt, R.I.G. Association Between Antipsychotic Medication Use and Diabetes. Curr. Diabetes Rep. 2019, 19, 96. [Google Scholar] [CrossRef]
- International Expert Committee. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009, 32, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.L.; Pyle, L.; Kelsey, M.; Newnes, L.; Zeitler, P.S.; Nadeau, K.J. Screening for type 2 diabetes and prediabetes in obese youth: Evaluating alternate markers of glycemia—1,5-anhydroglucitol, fructosamine, and glycated albumin. Pediatr. Diabetes 2016, 17, 206–211. [Google Scholar] [CrossRef]
- Gomez-Perez, F.J.; Aguilar-Salinas, C.A.; Almeda-Valdes, P.; Cuevas-Ramos, D.; Lerman Garber, I.; Rull, J.A. HbA1c for the diagnosis of diabetes mellitus in a developing country. A position article. Arch. Med. Res. 2010, 41, 302–308. [Google Scholar] [CrossRef]
- Ehehalt, S.; Wiegand, S.; Körner, A.; Schweizer, R.; Liesenkötter, K.P.; Partsch, C.J.; Blumenstock, G.; Spielau, U.; Denzer, C.; Ranke, M.B.; et al. Diabetes screening in overweight and obese children and adolescents: Choosing the right test. Eur. J. Pediatr. 2017, 176, 89–97. [Google Scholar] [CrossRef]
- Love-Osborne, K.A.; Sheeder, J.L.; Nadeau, K.J.; Zeitler, P. Longitudinal follow up of dysglycemia in overweight and obese pediatric patients. Pediatr. Diabetes 2018, 19, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.S.; So, C.H.; Lee, H.S.; Hwang, J.S. Glycated hemoglobin A1c as a screening test for detecting type 2 diabetes mellitus in obese children and adolescents. J. Pediatr. Endocrinol. Metab. 2018, 31, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Ni, J.; Su, H.; He, X.; Lu, W.; Zhu, W.; Wang, Y.; Ma, X.; Bao, Y.; Zhou, J. One-Hour Postload Glucose Is a More Sensitive Marker of Impaired β-Cell Function Than Two-Hour Postload Glucose. Diabetes 2025, 74, 36–42. [Google Scholar] [CrossRef]
- Ravà, L.; Fintini, D.; Mariani, M.; Deodati, A.; Inzaghi, E.; Pedicelli, S.; Bizzarri, C.; Cappa, M.; Cianfarani, S.; Manco, M. High 1-h glucose in youths with obesity as marker of prediabetes and cardiovascular risk. J. Endocrinol. Investig. 2023, 46, 2555–2562. [Google Scholar] [CrossRef]
- Harris, D.L.; Battin, M.R.; Weston, P.J.; Harding, J.E. Continuous glucose monitoring in newborn babies at risk of hypoglycemia. J. Pediatr. 2010, 157, 198–202.e1. [Google Scholar] [CrossRef]
- Agrawal, S.; Gensure, R. Commentary on the Impact of Obesity on PediatricDiabetes. Clin. Ther. 2018, 40, 1631–1637. [Google Scholar] [CrossRef]
- TODAY Study Group; Zeitler, P.; Hirst, K.; Pyle, L.; Linder, B.; Copeland, K.; Arslanian, S.; Cuttler, L.; Nathan, D.M.; Tollefsen, S.; et al. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N. Engl. J. Med. 2012, 366, 2247–2256. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.G.; Hsu, J.W.; Jahoor, F.; Coraza, I.; Bain, J.R.; Stevens, R.D.; Iyer, D.; Nalini, R.; Ozer, K.; Hampe, C.S.; et al. Pathogenesis of A−β+ ketosis-prone diabetes. Diabetes 2013, 62, 912–922. [Google Scholar] [CrossRef]
- Kubota-Mishra, E.; Huang, X.; Minard, C.G.; Astudillo, M.; Refaey, A.; Montes, G.; Sisley, S.; Ram, N.; Winter, W.E.; Naylor, R.N.; et al. High Prevalence of A-β+ Ketosis-Prone Diabetes in Children with Type 2 Diabetes and Diabetic Ketoacidosis at Diagnosis: Evidence from the Rare and Atypical Diabetes Network (RADIANT). Pediatr. Diabetes 2024, 2024, 5907924. [Google Scholar] [CrossRef]
- Muneer, M.; Akbar, I. Acute Metabolic Emergencies in Diabetes: DKA, HHS and EDKA. Adv. Exp. Med. Biol. 2021, 1307, 85–114. [Google Scholar]
- Zeitler, P.; Haqq, A.; Rosenbloom, A.; Glaser, N.; Drugs and Therapeutics Committee of the Lawson Wilkins Pediatric Endocrine Society. Hyperglycemic hyperosmolar syndrome in children: Pathophysiological considerations and suggested guidelines for treatment. J. Pediatr. 2011, 158, 9–14.e2. [Google Scholar] [CrossRef]
- Glaser, N.; Fritsch, M.; Priyambada, L.; Rewers, A.; Cherubini, V.; Estrada, S.; Wolfsdorf, J.I.; Codner, E. ISPAD clinical practice consensus guidelines 2022: Diabetic ketoacidosis and hyperglycemic hyperosmolar state. Pediatr. Diabetes 2022, 23, 835–856. [Google Scholar] [CrossRef]
- Curran, J.A.; Haynes, A.; Davis, E.A. Clinical characteristics of Western Australian children diagnosed with type 2 diabetes before 10 years of age. Med. J. Aust. 2020, 212, 95–95.e1. [Google Scholar] [CrossRef]
- Cioana, M.; Deng, J.; Nadarajah, A.; Hou, M.; Qiu, Y.; Chen, S.S.J.; Rivas, A.; Toor, P.P.; Banfield, L.; Thabane, L.; et al. Global Prevalence of Diabetic Retinopathy in Pediatric Type 2 Diabetes: A Systematic Review and Meta-analysis. JAMA Netw. Open 2023, 6, e231887. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, M.; Divers, J.; Dabelea, D.; Isom, S.; Bell, R.A.; Martin, C.L.; Pettitt, D.J.; Saydah, S.; Pihoker, C.; Standiford, D.A.; et al. Prevalence of and Risk Factors for Diabetic Peripheral Neuropathy in Youth with Type 1 and Type 2 Diabetes: SEARCH for Diabetes in Youth Study. Diabetes Care 2017, 40, 1226–1232. [Google Scholar] [CrossRef]
- Cooper, F.; Carakushansky, M.; Johnson, C.M.; Gurnurkar, S. Deep Vein Thrombosis as the Presenting Sign in an Adolescent with New-Onset Type 2 Diabetes. JCEM Case Rep. 2024, 2, luae038. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, J.; Cheng, P.; Ruedy, K.J.; Kollman, C.; Beck, R.W.; Klingensmith, G.J.; Wood, J.R.; Willi, S.; Bacha, F.; Lee, J.; et al. Depressive Symptoms in Youth with Type 1 or Type 2 Diabetes: Results of the Pediatric Diabetes Consortium Screening Assessment of Depression in Diabetes Study. Diabetes Care 2015, 38, 2341–2343. [Google Scholar] [CrossRef] [PubMed]
- Meehan, C.; Silverstein, J. Treatment Options for Type 2 Diabetes in Youth Remain Limited. J. Pediatr. 2016, 170, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Novotny, R.; Davis, J.; Butel, J.; Boushey, C.J.; Fialkowski, M.K.; Nigg, C.R.; Braun, K.L.; Guerrero, R.T.; Coleman, P.; Bersamin, A.; et al. Effect of the Children’s Healthy Living Program on Young Child Overweight, Obesity, and Acanthosis Nigricans in the US-Affiliated Pacific Region: A Randomized Clinical Trial. JAMA Netw. Open 2018, 1, e183896. [Google Scholar] [CrossRef]
- Pike, J.M.; Haberlin-Pittz, K.M.; Alharbi, B.S.; Perkins, S.M.; Hannon, T.S. A co-designed, community-based intensive health behavior intervention promotes participation and engagement in youth with risk factors for type 2 diabetes. Front. Clin. Diabetes Healthc. 2023, 4, 1264312. [Google Scholar] [CrossRef]
- Soltero, E.G.; Olson, M.L.; Williams, A.N.; Konopken, Y.P.; Castro, F.G.; Arcoleo, K.J.; Keller, C.S.; Patrick, D.L.; Ayers, S.L.; Barraza, E.; et al. Effects of a Community-Based Diabetes Prevention Program for Latino Youth with Obesity: A Randomized Controlled Trial. Obesity 2018, 26, 1856–1865. [Google Scholar] [CrossRef]
- Manios, Y.; Androutsos, O.; Lambrinou, C.P.; Cardon, G.; Lindstrom, J.; Annemans, L.; Mateo-Gallego, R.; de Sabata, M.S.; Iotova, V.; Kivela, J.; et al. A school- and community-based intervention to promote healthy lifestyle and prevent type 2 diabetes in vulnerable families across Europe: Design and implementation of the Feel4Diabetes-study. Public Health Nutr. 2018, 21, 3281–3290. [Google Scholar] [CrossRef]
- Cardon, G.; Chastin, S.; Van Stappen, V.; Huys, N.; Stefanova, T.; Chakarova, N.; Kivelä, J.; Alberto Moreno, L.; Sándor Istvánné, R.; Androutsos, O.; et al. The Feel4Diabetes intervention: Effectiveness on 24-hour physical behaviour composition in families at risk for diabetes development. Health Promot. Int. 2022, 37, daac092. [Google Scholar] [CrossRef]
- Van Stappen, V.; Latomme, J.; Cardon, G.; De Bourdeaudhuij, I.; Lateva, M.; Chakarova, N.; Kivelä, J.; Lindström, J.; Androutsos, O.; González-Gil, E.; et al. Barriers from Multiple Perspectives Towards Physical Activity, Sedentary Behaviour, Physical Activity and Dietary Habits When Living in Low Socio-Economic Areas in Europe. The Feel4Diabetes Study. Int. J. Environ. Res. Public Health 2018, 15, 2840. [Google Scholar] [CrossRef]
- Kriska, A.; El Ghormli, L.; Copeland, K.C.; Higgins, J.; Ievers-Landis, C.E.; Levitt Katz, L.E.; Trief, P.M.; Wauters, A.D.; Yasuda, P.M.; Delahanty, L.M.; et al. Impact of lifestyle behavior change on glycemic control in youth with type 2 diabetes. Pediatr. Diabetes 2018, 19, 36–44. [Google Scholar] [CrossRef]
- Berkowitz, R.I.; Marcus, M.D.; Anderson, B.J.; Delahanty, L.; Grover, N.; Kriska, A.; Laffel, L.; Syme, A.; Venditti, E.; Van Buren, D.J.; et al. Adherence to a lifestyle program for youth with type 2 diabetes and its association with treatment outcome in the TODAY clinical trial. Pediatr. Diabetes 2018, 19, 191–198. [Google Scholar] [CrossRef] [PubMed]
- McGavock, J.; Durksen, A.; Wicklow, B.; Malik, S.; Sellers, E.A.; Blydt-Hansen, T.; Chateau, D.; Dart, A. Determinants of Readiness for Adopting Healthy Lifestyle Behaviors Among Indigenous Adolescents with Type 2 Diabetes in Manitoba, Canada: A Cross-Sectional Study. Obesity 2018, 26, 910–915. [Google Scholar] [CrossRef] [PubMed]
- Ievers-Landis, C.E.; Walders-Abramson, N.; Amodei, N.; Drews, K.L.; Kaplan, J.; Levitt Katz, L.E.; Lavietes, S.; Saletsky, R.; Seidman, D.; Yasuda, P. Longitudinal Correlates of Health Risk Behaviors in Children and Adolescents with Type 2 Diabetes. J. Pediatr. 2015, 166, 1258–1264.e3. [Google Scholar] [CrossRef]
- Huynh, E.; Rand, D.; McNeill, C.; Brown, S.; Senechal, M.; Wicklow, B.; Dart, A.; Sellers, E.; Dean, H.; Blydt-Hansen, T.; et al. Beating Diabetes Together: A Mixed-Methods Analysis of a Feasibility Study of Intensive Lifestyle Intervention for Youth with Type 2 Diabetes. Can. J. Diabetes 2015, 39, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.A.; Erickson, E.; McKee, P.; Schrankler, K.; Raatz, S.K.; Lytle, L.A.; Pellegrini, A.D. Breakfast frequency and quality may affect glycemia and appetite in adults and children. J. Nutr. 2011, 141, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Gow, M.L.; Garnett, S.P.; Baur, L.A.; Lister, N.B. The Effectiveness of Different Diet Strategies to Reduce Type 2 Diabetes Risk in Youth. Nutrients 2016, 8, 486. [Google Scholar] [CrossRef]
- Diederichs, T.; Herder, C.; Roßbach, S.; Roden, M.; Wudy, S.A.; Nöthlings, U.; Alexy, U.; Buyken, A.E. Carbohydrates from Sources with a Higher Glycemic Index during Adolescence: Is Evening Rather than Morning Intake Relevant for Risk Markers of Type 2 Diabetes in Young Adulthood? Nutrients 2017, 9, 591. [Google Scholar] [CrossRef]
- Hegedus, E.; Vu, M.H.; Salvy, S.J.; Bakhsh, J.; Goran, M.I.; Raymond, J.K.; Espinoza, J.C.; Vidmar, A.P. Randomized Controlled Feasibility Trial of Late 8-Hour Time-Restricted Eating for Adolescents with Type 2 Diabetes. J. Acad. Nutr. Diet. 2024, 124, 1014–1028. [Google Scholar] [CrossRef]
- Stagi, S.; Lapi, E.; Seminara, S.; Pelosi, P.; Del Greco, P.; Capirchio, L.; Strano, M.; Giglio, S.; Chiarelli, F.; de Martino, M. Policaptil Gel Retard significantly reduces body mass index and hyperinsulinism and may decrease the risk of type 2 diabetes mellitus (T2DM) in obese children and adolescents with family history of obesity and T2DM. Ital. J. Pediatr. 2015, 41, 10. [Google Scholar] [CrossRef]
- Zhu, L.; Li, S.; Zhong, L.; Xu, S.; Zhu, H. Optimal vitamin D supplement dosage for improving insulin resistance in children and adolescents with overweight/obesity: A systematic review and network meta-analysis. Eur. J. Nutr. 2024, 63, 763–775. [Google Scholar] [CrossRef]
- Calcaterra, V.; Magenes, V.C.; Bianchi, A.; Rossi, V.; Gatti, A.; Marin, L.; Vandoni, M.; Zuccotti, G. How Can Promoting Skeletal Muscle Health and Exercise in Children and Adolescents Prevent Insulin Resistance and Type 2 Diabetes? Life 2024, 14, 1198. [Google Scholar] [CrossRef]
- Sénéchal, M.; Rempel, M.; Duhamel, T.A.; MacIntosh, A.C.; Hay, J.; Wicklow, B.; Wittmeier, K.; Shen, G.X.; McGavock, J.M. Fitness is a determinant of the metabolic response to endurance training in adolescents at risk of type 2 diabetes mellitus. Obesity 2015, 23, 823–832. [Google Scholar] [CrossRef]
- Lee, S.S.; Yoo, J.H.; So, Y.S. Effect of the low- versus high-intensity exercise training on endoplasmic reticulum stress and GLP-1 in adolescents with type 2 diabetes mellitus. J. Phys. Ther. Sci. 2015, 27, 3063–3068. [Google Scholar] [CrossRef]
- Colip, L.; Burge, M.R.; Sandy, P.; Ghahate, D.; Bobelu, J.; Faber, T.; Shah, V. Exercise Intervention Improves the Metabolic Profile and Body Composition of Southwestern American Indian Adolescents. J. Diabetes Obes. 2016, 3, 10-15436. [Google Scholar]
- Herbst, A.; Kapellen, T.; Schober, E.; Graf, C.; Meissner, T.; Holl, R.W.; DPV-Science-Initiative. Impact of regular physical activity on blood glucose control and cardiovascular risk factors in adolescents with type 2 diabetes mellitus—A multicenter study of 578 patients from 225 centres. Pediatr. Diabetes 2015, 16, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.J.; D’Angiulli, A.; Cameron, J.D.; Sigal, R.J.; Kenny, G.P.; Holcik, M.; Doucette, S.; Alberga, A.S.; Prud’hOmme, D.; Hadjiyannakis, S.; et al. Changes in the Brain-Derived Neurotrophic Factor Are Associated with Improvements in Diabetes Risk Factors after Exercise Training in Adolescents with Obesity: The HEARTY Randomized Controlled Trial. Neural Plast. 2018, 2018, 7169583. [Google Scholar] [CrossRef]
- Bacha, F.; Gidding, S.S.; Pyle, L.; Levitt Katz, L.; Kriska, A.; Nadeau, K.J.; Lima, J.A. Relationship of Cardiac Structure and Function to Cardiorespiratory Fitness and Lean Body Mass in Adolescents and Young Adults with Type 2 Diabetes. J. Pediatr. 2016, 177, 159–166.e1. [Google Scholar] [CrossRef] [PubMed]
- Naylor, L.H.; Davis, E.A.; Kalic, R.J.; Paramalingam, N.; Abraham, M.B.; Jones, T.W.; Green, D.J. Exercise training improves vascular function in adolescents with type 2 diabetes. Physiol. Rep. 2016, 4, e12713. [Google Scholar] [CrossRef] [PubMed]
- Chobot, A.; Piona, C.; Bombaci, B.; Kamińska-Jackowiak, O.; Mancioppi, V.; Passanisi, S. Exploring the Continuous Glucose Monitoring in Pediatric Diabetes: Current Practices, Innovative Metrics, and Future Implications. Children 2024, 11, 907. [Google Scholar] [CrossRef]
- Gonzalez, A.R.; Harrison, C.; Hewitt, B.; Mejier, J.L.; Vajravelu, M.E. Feasibility, Acceptability, and Validity of Home Continuous Glucose Monitoring-Based Oral Glucose Tolerance Test in Youth. J. Clin. Endocrinol. Metab. 2024, 110, e2510–e2516. [Google Scholar] [CrossRef]
- Chang, N.; Barber, R.O.L.B.; Llovido Alula, J.; Durazo-Arvizu, R.; Chao, L.C. Continuous Glucose Monitoring versus Standard of Care in Adolescents with Type 2 Diabetes: A Pilot Randomized Cross-Over Trial. J. Diabetes Sci. Technol. 2023, 17, 1419–1420. [Google Scholar] [CrossRef]
- Chesser, H.; Srinivasan, S.; Puckett, C.; Gitelman, S.E.; Wong, J.C. Real-Time Continuous Glucose Monitoring in Adolescents and Young Adults with Type 2 Diabetes Can Improve Quality of Life. J. Diabetes Sci. Technol. 2024, 18, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Manfredo, J.; Lin, T.; Gupta, R.; Abiola, K.; West, M.; Busin, K.; Tracey, J.; Brown, E.A.; Magge, S.N.; Wolf, R.M. Short-term use of CGM in youth onset type 2 diabetes is associated with behavioral modifications. Front. Endocrinol. 2023, 14, 1182260. [Google Scholar] [CrossRef] [PubMed]
- Alfaraidi, H.; Samaan, M.C. Metformin therapy in pediatric type 2 diabetes mellitus and its comorbidities: A review. Front. Endocrinol. 2022, 13, 1072879. [Google Scholar] [CrossRef]
- Pinto, C.A.; Stafford, J.M.; Wang, T.; Shankar, R.R.; Lawrence, J.M.; Kim, G.; Pihoker, C.; D’Agostino, R.B., Jr.; Dabelea, D. Changes in diabetes medication regimens and glycemic control in adolescents and young adults with youth-onset type 2 diabetes: The SEARCH for diabetes in youth study. Pediatr. Diabetes 2018, 19, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Kelsey, M.M.; Geffner, M.E.; Guandalini, C.; Pyle, L.; Tamborlane, W.V.; Zeitler, P.S.; White, N.H.; Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) Study Group. Presentation and effectiveness of early treatment of type 2 diabetes in youth: Lessons from the TODAY study. Pediatr. Diabetes 2016, 17, 212–221. [Google Scholar] [CrossRef]
- Bielinski, S.J.; Yanes Cardozo, L.L.; Takahashi, P.Y.; Larson, N.B.; Castillo, A.; Podwika, A.; De Filippis, E.; Hernandez, V.; Mahajan, G.J.; Gonzalez, C.; et al. Predictors of Metformin Failure: Repurposing Electronic Health Record Data to Identify High-Risk Patients. J. Clin. Endocrinol. Metab. 2023, 108, 1740–1746. [Google Scholar] [CrossRef]
- Bacha, F.; Cheng, P.; Gal, R.L.; Kollman, C.; Tamborlane, W.V.; Klingensmith, G.J.; Manseau, K.; Wood, J.; Beck, R.W.; Pediatric Diabetes Consortium. Initial Presentation of Type 2 Diabetes in Adolescents Predicts Durability of Successful Treatment with Metformin Monotherapy: Insights from the Pediatric Diabetes Consortium T2D Registry. Horm. Res. Paediatr. 2018, 89, 47–55. [Google Scholar] [CrossRef]
- Zeitler, P.; Hirst, K.; Copeland, K.C.; El Ghormli, L.; Levitt Katz, L.; Levitsky, L.L.; Linder, B.; McGuigan, P.; White, N.H.; Wilfley, D.; et al. HbA1c After a Short Period of Monotherapy with Metformin Identifies Durable Glycemic Control Among Adolescents with Type 2 Diabetes. Diabetes Care 2015, 38, 2285–2292. [Google Scholar] [CrossRef]
- Venditti, E.M.; Tan, K.; Chang, N.; Laffel, L.; McGinley, G.; Miranda, N.; Tryggestad, J.; Walders-Abramson, N.; Yasuda, P.; Delahanty, L. Barriers and strategies for oral medication adherence among children and adolescents with Type 2 diabetes. Diabetes Res. Clin. Pract. 2018, 139, 24–31. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, M.; Wen, Z.; Lu, Z.; Cui, L.; Fu, C.; Xue, H.; Liu, Y.; Zhang, Y. GLP-1 Receptor Agonists: Beyond Their Pancreatic Effects. Front. Endocrinol. 2021, 12, 721135. [Google Scholar] [CrossRef]
- Miller, M.G.; Terebuh, P.; Kaelber, D.C.; Xu, R.; Davis, P.B. Characterizing GLP-1 Receptor Agonist Use in Preadolescent and Adolescent Populations. JAMA Netw. Open 2024, 7, e2439887. [Google Scholar] [CrossRef]
- Dai, M.; Dai, S.; Gu, L.; Xiang, Z.; Xu, A.; Lu, S.; Yang, Y.; Zhou, C. Efficacy of Glucagon-like Peptide-1 Receptor Agonists in Overweight/Obese and/or T2DM Adolescents: A Meta-analysis Based on Randomized Controlled Trials. J. Clin. Res. Pediatr. Endocrinol. 2024, 16, 323–333. [Google Scholar] [CrossRef]
- Yugar, L.B.T.; Sedenho-Prado, L.G.; da Silva Ferreira, I.M.C.; Silva, C.A.M.; Sposito, A.C.; Cercato, C. The efficacy and safety of GLP-1 receptor agonists in youth with type 2 diabetes: A meta-analysis. Diabetol. Metab. Syndr. 2024, 16, 92. [Google Scholar] [CrossRef]
- Seo, J.Y.; Lee, C.G.; Choi, H.; Lee, H.K.; Lee, S.Y.; Kim, H.J.; Jung, K.Y.; Kim, J.T. Effects of once-weekly dulaglutide on juvenile type 2 diabetes mellitus and obesity in Korea: A pilot study. Ann. Pediatr. Endocrinol. Metab. 2023, 28, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Palmer, S.C.; Tendal, B.; Mustafa, R.A.; Vandvik, P.O.; Li, S.; Hao, Q.; Tunnicliffe, D.; Ruospo, M.; Natale, P.; Saglimbene, V.; et al. Sodium-glucose cotransporter protein-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists for type 2 diabetes: Systematic review and network meta-analysis of randomised controlled trials. BMJ 2021, 372, m4573. [Google Scholar] [CrossRef] [PubMed]
- Grube, P.M.; Beckett, R.D. Clinical studies of dapagliflozin in pediatric patients: A rapid review. Ann. Pediatr. Endocrinol. Metab. 2022, 27, 265–272. [Google Scholar] [CrossRef]
- Tirucherai, G.S.; LaCreta, F.; Ismat, F.A.; Tang, W.; Boulton, D.W. Pharmacokinetics and pharmacodynamics of dapagliflozin in children and adolescents with type 2 diabetes mellitus. Diabetes Obes. Metab. 2016, 18, 678–684. [Google Scholar] [CrossRef]
- Shehadeh, N.; Barrett, T.; Galassetti, P.; Karlsson, C.; Monyak, J.; Iqbal, N.; Tamborlane, W.V. Dapagliflozin or Saxagliptin in Pediatric Type 2 Diabetes. NEJM Evid. 2023, 2, EVIDoa2300210. [Google Scholar] [CrossRef] [PubMed]
- Shehadeh, N.; Galassetti, P.; Iqbal, N.; Karlsson, C.; Monyak, J.; Ostridge, J.; Bolin, M.; Barrett, T. Safety, Growth, and Development After Dapagliflozin or Saxagliptin in Children with Type 2 Diabetes (T2NOW Follow-Up). J. Clin. Endocrinol. Metab. 2025, 110, 1587–1595. [Google Scholar] [CrossRef]
- Tamborlane, W.V.; Polidori, D.; Argenti, D.; Di Prospero, N.A. Pharmacokinetics and pharmacodynamics of canagliflozin in pediatric patients with type 2 diabetes. Pediatr. Diabetes 2018, 19, 649–655. [Google Scholar] [CrossRef]
- Tamborlane, W.V.; Laffel, L.M.; Weill, J.; Gordat, M.; Neubacher, D.; Retlich, S.; Hettema, W.; Hoesl, C.E.; Kaspers, S.; Marquard, J. Randomized, double-blind, placebo-controlled dose-finding study of the dipeptidyl peptidase-4 inhibitor linagliptin in pediatric patients with type 2 diabetes. Pediatr. Diabetes 2018, 19, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Fraser, I.P.; Neufeld, N.D.; Fox, L.A.; Kipnes, M.S.; Miller, T.L.; Zeitler, P.S.; Rodriguez, H.; Gilmartin, J.H.; Lee, S.J.; Patterson, J.K.; et al. A randomized clinical trial to evaluate the single-dose pharmacokinetics, pharmacodynamics, and safety of sitagliptin in pediatric patients with type 2 diabetes. Pediatr. Diabetes 2019, 20, 48–56. [Google Scholar] [CrossRef] [PubMed]
- RISE Consortium. Impact of Insulin and Metformin Versus Metformin Alone on β-Cell Function in Youth with Impaired Glucose Tolerance or Recently Diagnosed Type 2 Diabetes. Diabetes Care 2018, 41, 1717–1725. [Google Scholar] [CrossRef]
- Shahid, M.; Shaibi, G.Q.; Baines, H.; Garcia-Filion, P.; Gonzalez-Garcia, Z.; Olson, M. Risk of hypoglycemia in youth with type 2 diabetes on insulin. J. Pediatr. Endocrinol. Metab. 2018, 31, 625–630. [Google Scholar] [CrossRef]
- Bally, L.; Thabit, H.; Hartnell, S.; Andereggen, E.; Ruan, Y.; Wilinska, M.E.; Evans, M.L.; Wertli, M.M.; Coll, A.P.; Stettler, C.; et al. Closed-Loop Insulin Delivery for Glycemic Control in Noncritical Care. N. Engl. J. Med. 2018, 379, 547–556. [Google Scholar] [CrossRef]
- Shah, A.S.; D’Alessio, D.; Ford-Adams, M.E.; Desai, A.P.; Inge, T.H. Bariatric Surgery: A Potential Treatment for Type 2 Diabetes in Youth. Diabetes Care 2016, 39, 934–940. [Google Scholar] [CrossRef][Green Version]
- Beamish, A.J. Bariatric surgery for obese adolescents to prevent type 2 diabetes. BMJ 2016, 353, i2977. [Google Scholar] [CrossRef]
- Inge, T.H.; Laffel, L.M.; Jenkins, T.M.; Marcus, M.D.; Leibel, N.I.; Brandt, M.L.; Haymond, M.; Urbina, E.M.; Dolan, L.M.; Zeitler, P.S. Comparison of Surgical and Medical Therapy for Type 2 Diabetes in Severely Obese Adolescents. JAMA Pediatr. 2018, 172, 452–460. [Google Scholar] [CrossRef]
- Asiri, A.; Alzahrani, F.; Alghamdi, H.; Alamri, Z. Effect of Laparoscopic Sleeve Gastrectomy on HbA1C Level in Children with Type 2 Diabetes Mellitus. Medicina 2022, 58, 959. [Google Scholar] [CrossRef] [PubMed]
- Carbajo, M.A.; Gonzalez-Ramirez, G.; Jimenez, J.M.; Luque-de-Leon, E.; Ortiz-de-Solorzano, J.; Castro, M.J.; Luque-De-Leon, E.; Ortiz-De-Solorzano, J.; Castro, M.J.; Ruiz-Tovar, J. A 5-Year Follow-up in Children and Adolescents Undergoing One-Anastomosis Gastric Bypass (OAGB) at a European IFSO Excellence Center (EAC-BS). Obes. Surg. 2019, 29, 2739–2744. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).