Permanence of Cognitive Alterations in Post- and Long COVID Patients: Glia and Brain Alteration, Gender Differences and New Diabetes Diagnosis
Abstract
1. Introduction
- The potential presence of encephalic alterations—from the cortex to the brain stem—detected through state-of-the-art neuroimaging techniques as previously explored by Crunfli in 2022.
- The possible correlations between these neurological changes and neurocognitive deficits, with specific attention to alterations in diabetic patients (types 1 and 2).
- The hypothesis that neuroinflammation, a condition exacerbated by COVID-19 as confirmed by recent findings [2], may lead to severe executive dysfunctions, akin to a dysexecutive syndrome.
2. Methods
2.1. Patients
2.2. Phases of the Research
2.3. Instruments
3. Multivariate Analysis
4. Results and Discussion
4.1. Cognitive Alteration
- Mild Cognitive Impairment: Detected in 47% (p < 0.05; 95%) of participants and higher in females (31% (p < 0.05; 95%) female, 16% (p < 0.05; 95%) male). This is the largest category. It indicates that nearly half of the post-COVID patients have experienced mild cognitive alterations. This reflects how the virus can impact cognitive functions, albeit not severely, and underscores the importance of monitoring even subtle changes over time.
- Moderate to Severe Impairment: Noted in 21.6% (p < 0.05; 95%) of the male and 12% (p < 0.05; 95%) of the female patients, respectively, with a significant prevalence in males. This category demonstrates that a significant segment of the population experiences cognitive impacts that extend beyond mild. These patients may require more intensive supportive interventions to address these challenges.
- Severe Deficits: 10.8% (p < 0.05; 95%) of the study population showed severe deficits, signaling that this group of individuals may face substantial daily life challenges due to their post-COVID neurological conditions.
- Individual Domain Alterations: At 26.4% (p < 0.05; 95%), this suggests there is a considerable percentage of individuals with disorders manifesting in specific cognitive domains, such as memory, attention, or other executive functions.
- Absence of Decline: Although it represents a small slice of the chart at 5.0% (p < 0.05; 95%), this category is important to acknowledge because it shows that a portion of the patients has not exhibited any detectable cognitive decrease based on the tests used. However, it is noteworthy that the sum of the percentages exceeds 100% (p < 0.05; 95%), which might indicate an overlap of categories (for instance, some individuals may have more than one type of deficit) [7,15,16].
4.2. PTSD Symptoms
- Mild Cognitive Deficits: 33.6% (p < 0.05; 95%) of patients who exhibited mild cognitive impairments also showed symptoms of PTSD. This high percentage suggests that a substantial majority of those experiencing less severe cognitive issues are also dealing with significant psychological stress.
- Severe Cognitive Disorders: 20.4% (p < 0.05; 95%) of patients with severe cognitive impairments had PTSD symptoms. While lower than the percentage for mild cognitive deficits, this still represents more than half of the patients with severe cognitive issues, indicating a considerable overlap between severe cognitive decline and PTSD.
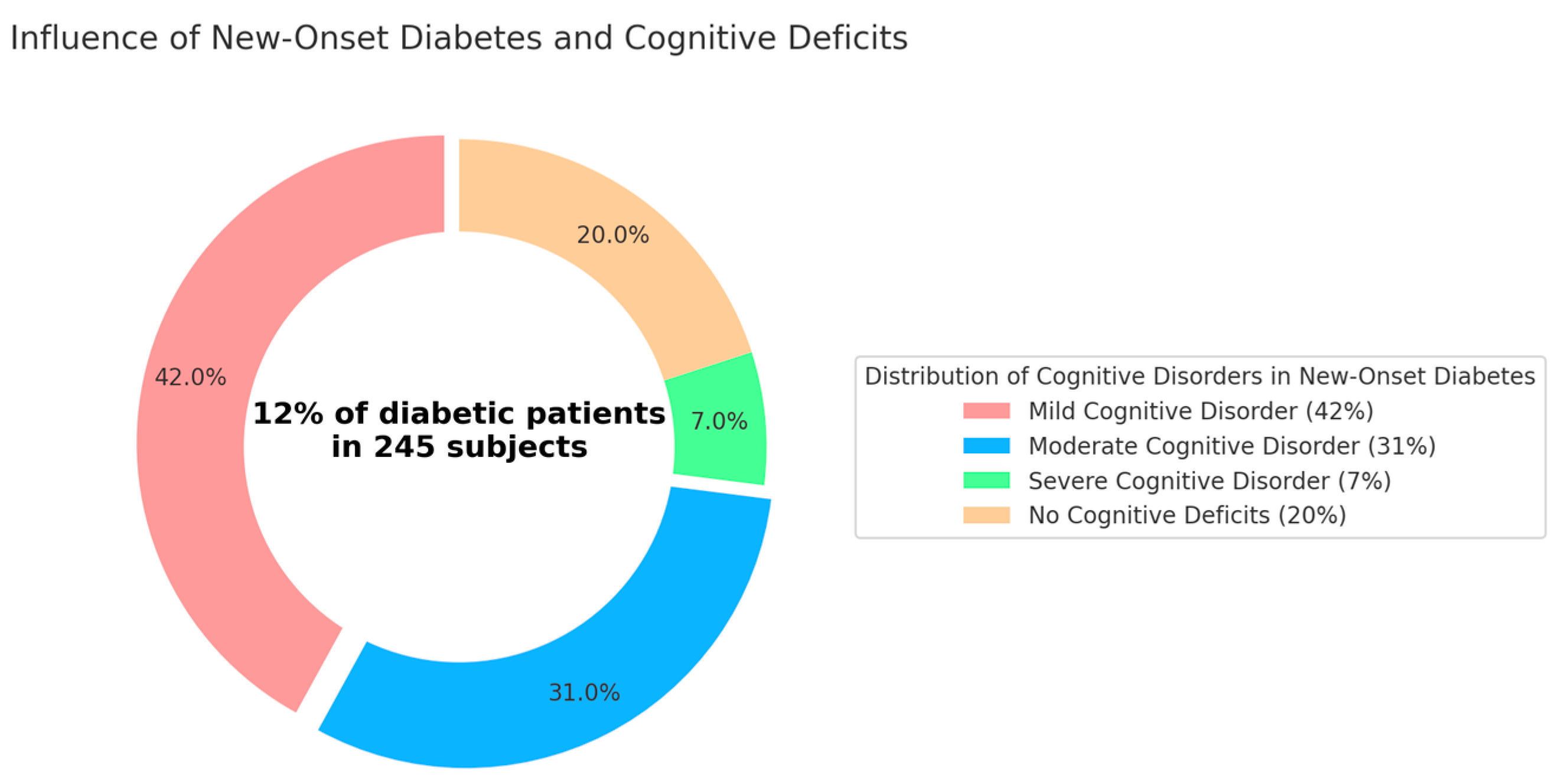
4.3. New-Onset Diabetes
- -
- Mild Cognitive Disorder: 42% (p < 0.05; 95%) of new-onset diabetic patients.
- -
- Moderate Cognitive Disorder: 31% (p < 0.05; 95%) of new-onset diabetic patients.
- -
- Severe Cognitive Disorder: 7% (p < 0.05; 95%) of new-onset diabetic patients.
- -
- No Cognitive Deficits: Surprisingly, 20% (p < 0.05; 95%) of the patients exhibited no cognitive deficits, suggesting that a portion of new-onset diabetic patients maintain normal cognitive functions. In Italy, the percentage of people with diabetes stands at 6% (p < 0.05; 95%), with 1.8% (p < 0.05; 95%) having type 1 diabetes. Analyzing the age groups of individuals with diabetes revealed that almost 65% (p < 0.05; 95%) (63.5% (p < 0.05; 95%)) are aged 65 or older, and 20% (p < 0.05; 95%) of Italian diabetics are 80 years old or more. Working-age individuals between 20 and 64 years old represent a significant portion, accounting for 35% (p < 0.05; 95%) (precisely 34.6% (p < 0.05; 95%), approximately one million people). Among these, the largest share belongs to the higher age bracket (50–64 years), comprising 23.7% (p < 0.05; 95%) of all Italian diabetics. Younger individuals, under 20 years of age, make up about 2% (p < 0.05; 95%) of the total.
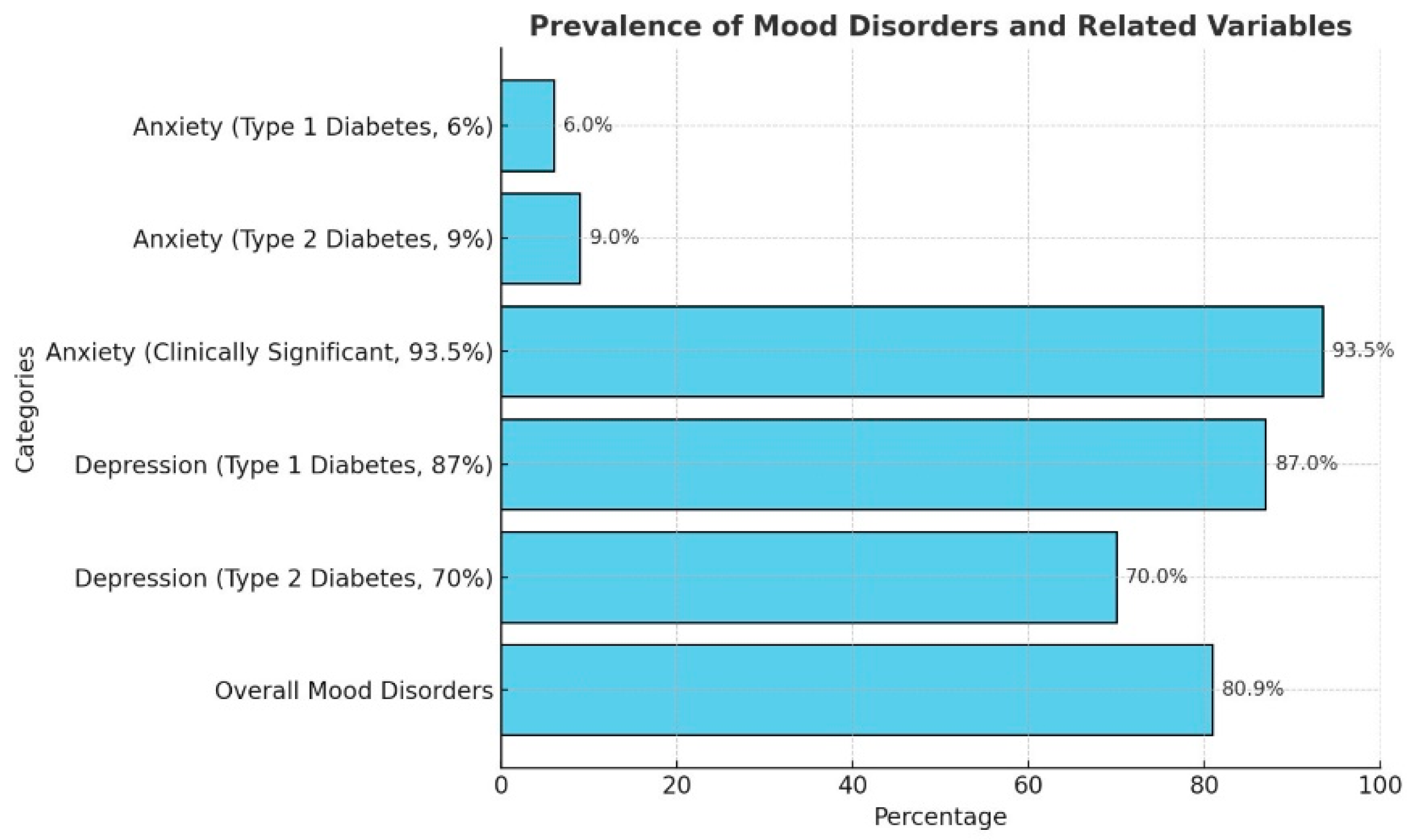
4.4. Mood Disorders: Anxiety and Affective Disorders in Diabetes
- These findings underscore the necessity for a comprehensive and integrated approach to post-COVID care, addressing both cognitive and psychological health. Tailored therapeutic strategies, informed by ongoing research and international collaboration, are essential for managing the diverse and interconnected sequelae of COVID-19. Continued research and targeted interventions will be pivotal in navigating the long-term health implications of the pandemic and ensuring effective patient care.
4.5. MRI Findings in Post- and Long COVID
- Our study revealed significant alterations in the MRIs of post- and long COVID patients, highlighting a range of brain changes, which are in agreement with the findings of researchers Marius Schwabenland and Henrike Salié as published in the journal Immunology. Their work underscores the neuroinflammatory processes and immune responses triggered by SARS-CoV-2, which may contribute to the observed structural and functional changes in the brain [29,30].
4.5.1. Glial Alterations
4.5.2. Temporal Lobe Atrophy
4.5.3. Reduced Hippocampal Size
4.6. Limitations and Strengths of This Study
5. Conclusions
5.1. Psychological Sequelae
5.2. Intersection with Metabolic Health
5.3. Neuroanatomy Sequela
5.4. Conclusions Enhanced by Global Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lauria, A.; Carfì, A.; Benvenuto, F.; Bramato, G.; Ciciarello, F.; Rocchi, S.; Rota, E.; Salerno, A.; Stella, L.; Tritto, M.; et al. Gemelli Against COVID-19 Post-acute Care Group m Neuropsychological measures of post-COVID-19 cognitive status. Front. Psychol. 2023, 14, 1136667. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Morawa, E.; Krehbiel, J.; Borho, A.; Herold, R.; Lieb, M.; Schug, C.; Erim, Y. Cognitive impairments and mental health of patients with post-COVID-19: A cross-sectional study. J. Psychosom. Res. 2023, 173, 111441. [Google Scholar] [CrossRef] [PubMed]
- The Lancet Neurology. The neurological impact of COVID-19. Lancet Neurol. 2020, 19, 471. [Google Scholar] [CrossRef]
- Crunfli, F.; Carregari, V.C.; Veras, F.P.; Silva, L.S.; Nogueira, M.H.; Antunes, A.S.L.M.; Vendramini, P.H.; Valença, A.G.F.; Brandão-Teles, C.; Zuccoli, G.d.S.; et al. Morphological, cellular, and molecular basis of brain infection in COVID-19 patients. Proc. Natl. Acad. Sci. USA 2022, 119, e2200960119. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schwabenland, M.; Salié, H.; Tanevski, J.; Killmer, S.; Lago, M.S.; Schlaak, A.E.; Mayer, L.; Matschke, J.; Püschel, K.; Fitzek, A.; et al. Deep spatial profiling of human COVID-19 brains reveals neuroinflammation with distinct microanatomical microglia-T-cell interactions. Immunity 2021, 54, 1594–1610.e11. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Welcome, M.O.; Mastorakis, N.E. Neuropathophysiology of coronavirus disease 2019: Neuroinflammation and blood brain barrier disruption are critical pathophysiological processes that contribute to the clinical symptoms of SARS-CoV-2 infection. Inflammopharmacology 2021, 29, 939–963. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mezzatesta, C.; Bazzano, S.; Gesualdo, R.; Marchese, S.; Savona, M.L.; Reyes, M.T.; Provenzano, V. Neurocognitive Disorders in Post and Long Covid Patients: Preliminary Data, Gender Differences and New Diabetes Diagnosis. Diabetology 2022, 3, 514–523. [Google Scholar] [CrossRef]
- Di Stadio, A.; Brenner, M.J.; De Luca, P.; Albanese, M.; D'Ascanio, L.; Ralli, M.; Roccamatisi, D.; Cingolani, C.; Vitelli, F.; Camaioni, A.; et al. Olfactory Dysfunction, Headache, and Mental Clouding in Adults with Long-COVID-19: What Is the Link between Cognition and Olfaction? A Cross-Sectional Study. Brain Sci. 2022, 12, 154. [Google Scholar] [CrossRef]
- Sathish, T.; Kapoor, N.; Cao, Y.; Tapp, R.J.; Zimmet, P. Proportion of newly diagnosed diabetes in COVID-19 patients: A systematic review and meta-analysis. Diabetes Obes. Metab. 2021, 23, 870–874. [Google Scholar] [CrossRef]
- Ellul, M.A.; Benjamin, L.; Singh, B.; Lant, S.; Michael, B.D.; Easton, A.; Kneen, R.; Defres, S.; Sejvar, J.; Solomon, T. Neurological associations of COVID-19. Lancet Neurol. 2020, 19, 767–783. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shukla, N.; Roelle, S.M.; Suzart, V.G.; Bruchez, A.M.; Matreyek, K.A. Mutants of human ACE2 differentially promote SARS-CoV and SARS-CoV-2 spike mediated infection. PLoS Pathog. 2021, 17, e1009715. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ilias, I.; Jahaj, E.; Kokkoris, S.; Zervakis, D.; Temperikidis, P.; Magira, E.; Pratikaki, M.; Vassiliou, A.G.; Routsi, C.; Kotanidou, A. Clinical Study of Hyperglycemia and SARS-CoV-2 Infection in Intensive Care Unit Patients. Vivo 2020, 34, 3029–3032. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Denes, G. Manuale di Neuropsicologia. Normalità e Patologia dei Processi Cognitivi; Zanichelli: Bologna, Italy, 2019. [Google Scholar]
- Joseph, S. Psychometric Evaluation of Horowitz's Impact of Event Scale: A Review. J. Trauma. Stress 2000, 13, 101–113. [Google Scholar] [CrossRef]
- Steenblock, C.; Schwarz, P.E.H.; Perakakis, N.; Brajshori, N.; Beqiri, P.; Bornstein, S.R. The interface of COVID-19, diabetes, and depression. Discov. Ment. Health 2022, 2, 5. [Google Scholar] [CrossRef] [PubMed]
- Hey, J.A.; Abushakra, S.; Blennow, K.; Reiman, E.M.; Hort, J.; Prins, N.D.; Sheardova, K.; Kesslak, P.; Shen, L.; Zhu, X.; et al. Effects of Oral ALZ-801/Valiltramiprosate on Plasma Biomarkers, Brain Hippocampal Volume, and Cognition: Results of 2-Year Single-Arm, Open-Label, Phase 2 Trial in APOE4 Carriers with Early Alzheimer’s Disease. Drugs 2024, 84, 811–823. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zerach, G.; Levi-Belz, Y. Moral injury, PTSD, and complex PTSD among Israeli health and social care workers during the COVID-19 pandemic: The moderating role of self-criticism. Psychol. Trauma Theory Res. Pract. Policy 2022, 14, 1314–1323. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.U.; Lustigova, E.; Chen, Q.; Dong, E.Y.; Maitra, A.; Chari, S.T.; Feng, Z.; Rinaudo, J.A.; Matrisian, L.M.; Parker, R.A. Consortium for the Study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer (CPDPC). Imaging of the Pancreas in New-Onset Diabetes: A Prospective Pilot Study. Clin. Transl. Gastroenterol. 2022, 13, e00478. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zubair, A.S.; McAlpine, L.S.; Gardin, T.; Farhadian, S.; Kuruvilla, D.E.; Spudich, S. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: A review. JAMA Neurol. 2020, 77, 1018–1027. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Mu, J.; Guo, J.; Lu, L.; Liu, D.; Luo, J.; Li, N.; Liu, J.; Yang, D.; Gao, H.; et al. New onset neurologic events in people with COVID-19 in 3 regions in China. Neurology 2020, 95, e1479–e1487. [Google Scholar] [CrossRef] [PubMed]
- Helms, J.; Kremer, S.; Merdji, H.; Schenck, M.; Severac, F.; Clere-Jehl, R.; Studer, A.; Radosavljevic, M.; Kummerlen, C.; Monnier, A.; et al. Delirium and encephalopathy in severe COVID-19: A cohort analysis of ICU patients. Crit. Care 2020, 24, 491. [Google Scholar] [CrossRef] [PubMed]
- Herman, C.; Mayer, K.; Sarwal, A. Scoping review of prevalence of neurologic comorbidities in patients hospitalized for COVID-19. Neurology 2020, 95, 77–84. [Google Scholar] [CrossRef]
- Pleasure, S.J.; Green, A.J.; Josephson, S.A. The spectrum of neurologic disease in the severe acute respiratory syndrome coronavirus 2 pandemic infection: Neurologists move to the frontlines. JAMA Neurol. 2020, 77, 679–680. [Google Scholar] [CrossRef]
- Beyrouti, R.; Adams, M.E.; Benjamin, L.; Cohen, H.; Farmer, S.F.; Goh, Y.Y.; Humphries, F.; Jäger, H.R.; Losseff, N.A.; Perry, R.J.; et al. Characteristics of ischaemic stroke associated with COVID-19. J. Neurol. Neurosurg. Psychiatry 2020, 91, 889–891. [Google Scholar] [CrossRef] [PubMed]
- Zanin, L.; Saraceno, G.; Panciani, P.P.; Renisi, G.; Signorini, L.; Migliorati, K.; Fontanella, M.M. SARS-CoV-2 can induce brain and spine demyelinating lesions. Acta Neurochir. 2020, 162, 1491–1494. [Google Scholar] [CrossRef]
- Varatharaj, A.; Thomas, N.; Ellul, M.A.; Davies, N.W.S.; Pollak, T.A.; Tenorio, E.L.; Sultan, M.; Easton, A.; Breen, G.; Zandi, M.; et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: A UK-wide surveillance study. Lancet Psychiatry 2020, 7, 875–882. [Google Scholar] [CrossRef]
- Benussi, A.; Pilotto, A.; Premi, E.; Libri, I.; Giunta, M.; Agosti, C.; Alberici, A.; Baldelli, E.; Benini, M.; Bonacina, S.; et al. Clinical characteristics and outcomes of inpatients with neurologic disease and COVID-19 in Brescia, Lombardy, Italy. Neurology 2020, 95, e910–e920. [Google Scholar] [CrossRef]
- Alemanno, F.; Houdayer, E.; Parma, A.; Spina, A.; Del Forno, A.; Scatolini, A.; Angelone, S.; Brugliera, L.; Tettamanti, A.; Beretta, L.; et al. COVID-19 cognitive deficits after respiratory assistance in the subacute phase: A COVID-rehabilitation unit experience. PLoS ONE 2021, 16, e0246590. [Google Scholar] [CrossRef]
- Beghi, E.; Helbok, R.; Crean, M.; Chou, S.H.; McNett, M.; Moro, E.; Bassetti, C.; Jenkins, T.; Oertzen, T.; Bodini, B.; et al. EAN Neuro-COVID Task Force. The European Academy of Neurology COVID-19 registry (ENERGY): An international instrument for surveillance of neurological complications in patients with COVID-19. Eur. J. Neurol. 2020, 28, 3303–3323. [Google Scholar] [CrossRef]
- Zádori, N.; Váncsa, S.; Farkas, N.; Hegyi, P.; Erőss, B.; KETLAK Study Group. The negative impact of comorbidities on the disease course of COVID-19. Intensiv. Care Med. 2020, 46, 1784–1786. [Google Scholar] [CrossRef] [PubMed]
- Kandel, E.R.; Schwartz, J.H.; Jessell, T.M.; Siegelbaum, S.A.; Hudspeth, A.J.; Perri, V.; Spidalieri, G. Principi di Neuroscienze; Casa Editrice Ambrosiana: Milano, Italy, 2014. [Google Scholar]
- Sammarra, I.; Martino, I.; Caligiuri, M.E.; Giugno, A.; Fortunato, F.; Labate, A.; Gambardella, A. The impact of one-year COVID-19 containment measures in patients with mesial temporal lobe epilepsy: A longitudinal survey-based study. Epilepsy Behav. 2022, 128, 108600. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yong, S.J. Long COVID or post-COVID-19 syndrome: Putative pathophysiology, risk factors, and treatments. Infect. Dis. 2021, 53, 737–754. [Google Scholar] [CrossRef] [PubMed]
- Fine, J.S.; Ambrose, A.F.; Didehbani, N.; Fleming, T.K.; Glashan, L.; Longo, M.; Merlino, A.; Ng, R.; Nora, G.J.; Rolin, S.; et al. Multi-disciplinary collaborative consensus guidance statement on the assessment and treatment of cognitive symptoms in patients with post-acute sequelae of SARS-CoV-2 infection (PASC). PM&R 2022, 14, 96–111. [Google Scholar] [CrossRef]
- Gupta, A.; Duggal, R. Incidence of New-onset Hypertension and New-onset Type 2 Diabetes during or after SARS-CoV-2 Infection. J. Assoc Physicians India. 2023, 71, 78–82. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mezzatesta, C.; Brancato, D.; Provenzano, F.; Marchese, S.; Savona, M.L.; Bazzano, S.; Gesualdo, R.; Cannia, F.; Porcino, A.E.; Reyes, M.T.; et al. Permanence of Cognitive Alterations in Post- and Long COVID Patients: Glia and Brain Alteration, Gender Differences and New Diabetes Diagnosis. Diabetology 2025, 6, 86. https://doi.org/10.3390/diabetology6090086
Mezzatesta C, Brancato D, Provenzano F, Marchese S, Savona ML, Bazzano S, Gesualdo R, Cannia F, Porcino AE, Reyes MT, et al. Permanence of Cognitive Alterations in Post- and Long COVID Patients: Glia and Brain Alteration, Gender Differences and New Diabetes Diagnosis. Diabetology. 2025; 6(9):86. https://doi.org/10.3390/diabetology6090086
Chicago/Turabian StyleMezzatesta, Concetta, Davide Brancato, Francesca Provenzano, Simone Marchese, Maria Luisa Savona, Sara Bazzano, Rosa Gesualdo, Francesco Cannia, Angela Eleonora Porcino, Mario Tambone Reyes, and et al. 2025. "Permanence of Cognitive Alterations in Post- and Long COVID Patients: Glia and Brain Alteration, Gender Differences and New Diabetes Diagnosis" Diabetology 6, no. 9: 86. https://doi.org/10.3390/diabetology6090086
APA StyleMezzatesta, C., Brancato, D., Provenzano, F., Marchese, S., Savona, M. L., Bazzano, S., Gesualdo, R., Cannia, F., Porcino, A. E., Reyes, M. T., & Provenzano, V. (2025). Permanence of Cognitive Alterations in Post- and Long COVID Patients: Glia and Brain Alteration, Gender Differences and New Diabetes Diagnosis. Diabetology, 6(9), 86. https://doi.org/10.3390/diabetology6090086





